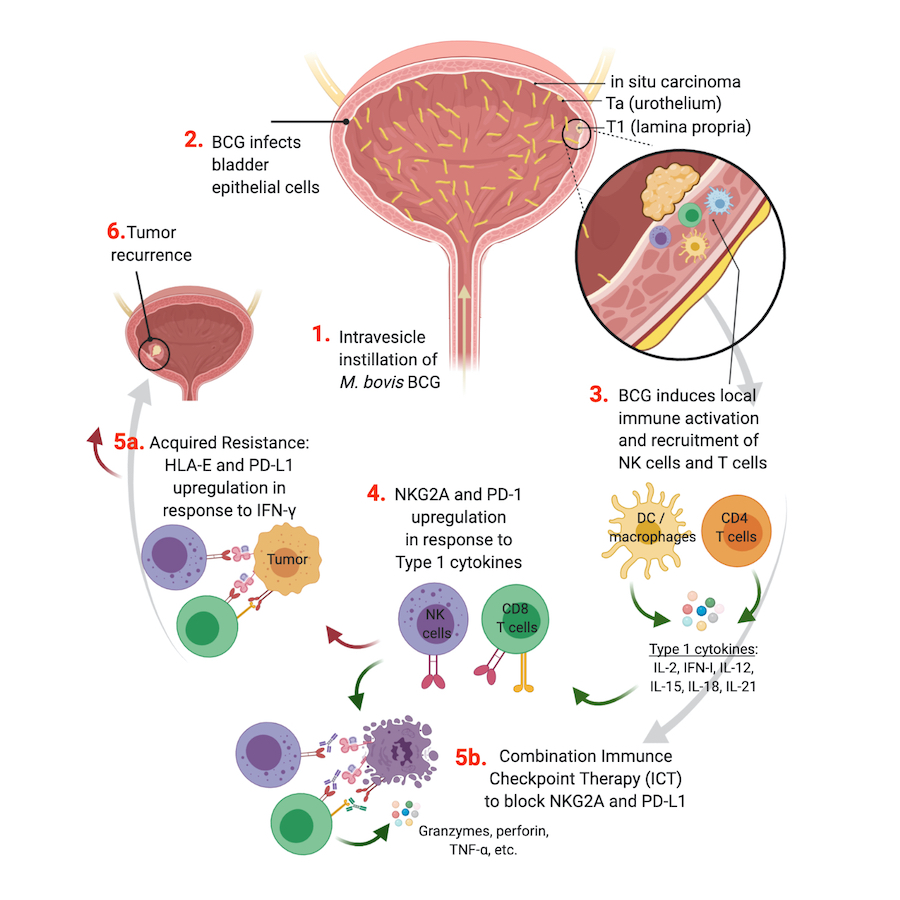
Model of tumor resistance to intravesicle BCG therapy and strategies to harness NK cell and CD8 T cell anti-tumor functions.
With 180,500 new cases diagnosed each year worldwide, bladder cancer is the 5th most common cancer in the United States. 75% of bladder tumors are non-muscle invasive (NMIBC) and 25% are muscle-invasive (MIBC) or metastatic. Transurethral resection of bladder tumor (TURBT) is performed on NMIBC patients, followed by intravesical instillation of Mycobacterium bovis Bacillus Calmette-Guérin (BCG) (Fig.1, step-1). Recurrence after BCG therapy will occur in 32-42% patients and tumor progression in 13% patients. BCG most readily infects the bladder urothelial cells (Fig.1, step-2), leading to a local pro-inflammatory response and immune cell recruitment within the tumor (Fig.1, step-3).
Immune cell activation is regulated by a balance between activating and inhibitory signals (e.g immune checkpoints). Immune checkpoints reduce the anti-tumor activity of effector cells, contributing to their exhaustion and subsequent tumor progression. Preliminary data from the Horowitz Lab indicates that BCG-induced activation drives increased expression of PD-1 and NKG2A on CD8 T cells and NKG2A on NK cells and recruitment of these activated cells to the TME (Fig.1, step-4). Upon infiltration into the tumor, activated NK and CD8 T cells secrete large amounts of IFN-g that act on tumors to increase expression of PD-L1 and HLA-E leading to strong inhibition of both effector cell subsets and uncontrolled recurrence of tumor (Fig. 1, step-5a). Four stages of T cell exhaustion have been described, with terminally exhausted cells displaying an irreversible anergic profile. NKG2A is an immune checkpoint that inhibits NK and CD8 T cell functions upon interaction of the CD94/NKG2A heterodimer with HLA-E.
Dr. Horowitz has published extensively on the immunogenetics underlying HLA-E expression and its regulatory effects on NKG2A-mediated function. NKG2A expression is associated with poor survival in numerous cancer types. The anti-NKG2A blocking antibody, monalizumab, can restore NK and CD8 T cell cytotoxicity when co-cultured in vitro with HLA-E+ cell lines. Other groups have demonstrated pre-clinical mouse models with a promising effect of monalizumab on survival in lung cancer and lymphoma. Monalizumab is currently under clinical trials in combination with ibrutinib in chronic lymphocytic leukemia (NCT02557516), with trastuzumab in breast cancer (NCT04307329), with durvalumab in colorectal cancer (NCT02671435) and with cetuximab in HNSCC (NCT02643550).
Innovation: This project is pursuing the identification of biomarkers of primary response and resistance to BCG and NMIBC and the underlying mechanisms. Key to achieving our overall goal is the integration of expertise in cancer biology, immunology, infectious disease, molecular biology, and epidemiology. Our team, workflow, assembled clinical cohorts, resources, and preliminary data are in each instance innovative and together could result in a significant advance in BCG immunotherapy and identification of novel treatment strategies for NMIBC.
In this study, the Horowitz Lab is collaborating with Dr. John Sfakianos (Urology), Dr. Nina Bhardwaj (Medicine), Dr. Matthew Galsky (Urology), Dr. Benjamin Hopkins (Oncological Sciences), and Anna Tocheva (Genetics and Genomic Sciences) at the Icahn School of Medicine at Mount Sinai. Additionally, this study involves external collaboration with Dr. Emily Mace (Columbia University) and Astra Zeneca for use of monalizumab and durvalumab.
Trainees involved on this projects: Dr. Jorge Daza, MD, Yuan-Sho (Alice) Wang, PhD (3rd YR medical student), Daniel Ranti (3rd YR medical student), Dr. Bérengère Salomé, PharmD, PhD
