ADAM FARKAS PHD AND DINA YOUSSEF
Natural Killer (NK) cells are potently cytolytic innate lymphocytes that play important roles in both anti-viral immunity, and tumor immunosurveillance. Unlike T cells, NK cells lack a somatically recombined, antigen-specific receptor, and therefore do not recognize specific peptides presented by putative target cells. Rather, target recognition relies on down-regulation of Class I HLA, and up-regulation of molecules associated with cellular stress (e.g. MICA/MICB, and the polio virus receptor CD155), both of which are hallmarks of virally-infected and transformed cells. NK cell recognition of targets is further tuned by integrating signals transmitted by a diverse array of activating (e.g. NKG2D, CD16, CD226, NKp44/NKp46) and inhibitory (e.g. KIR molecules, NKG2A, Tim-3 and TIGIT) receptors. Additionally, a major role for NK cells in attracting and maintaining dendritic cells (DC) to the tumor site has been appreciated, highlighting their importance in facilitating the ensuing T cell response, in addition to their intrinsic tumoricidal roles1,2.
Given their ability to recognize and destroy tumor cells, our lab and others are focused on understanding the molecular mechanisms by which NK cells fail to adequately control tumors. In the context of advanced melanoma, we found that NK cells undergo a parallel process of “exhaustion”, analogous to that of T cells3. Peripheral blood NK cells from Stage III/IV melanoma patients failed to proliferate, produce the tumoricidal cytokine interferon gamma (IFNγ), or kill tumor cells as well as NK cells from healthy donors (HD). In particular we found that Tim-3, an inhibitory receptor expressed by both NK cells and T cells, was highly up-regulated by NK cells from melanoma patients, and its blockade using a Tim-3 Ab restored anti-tumor effector function to exhausted NK cells (Figure 1). Similarly, we have found that melanoma patients who respond to ICB against CTLA-4 exhibit restored NK cell function, despite the fact that NK cells express very little CTLA-4. This result highlights the potential importance of dissecting the cross-talk between innate and adaptive lymphocytes involved in tumor immunosurveillance.
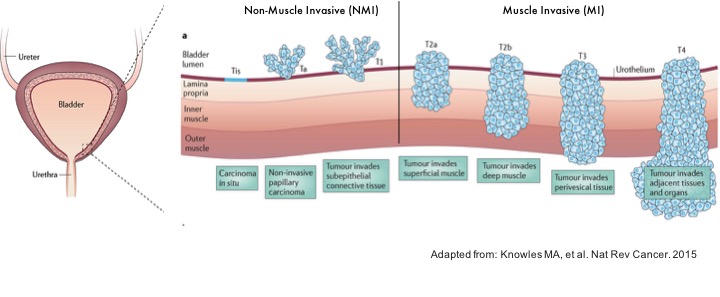 More recently, we have examined differences in NK cell phenotype and function in patients with bladder cancer (BlCa) with the goal of designing immunotherapies that can overcome the suppressive milieu of the tumor microenvironment (TME)4. BlCa is the 6thmost commonly diagnosed cancer in the United States, as well as having the 6thhighest mortality. Clinically, BlCa is divided into early, non-muscle-invasive tumors (NMI), and more advanced muscle-invasive (MI) tumors5(Figure 2), and a subset of patients with metastatic and chemo-ineligible BlCa are among those for whom T cell-centric ICB has shown benefit. Using flow cytometry (FACS), and mass cytometry (CyTOF), we found that bladder tumors were well-infiltrated by NK cells, which represented the second most frequent immune lineage after CD4+and CD8+T cells. However, MI tumors had significantly fewer NK cells than NMI tumors, suggesting a role for NK cells in controlling tumor progression. We found that both NK and T cells significantly up-regulate the inhibitory receptors Tim-3 and TIGIT in both the peripheral blood and in primary tumor tissue. This over-expression occurs in both major subsets of human NK cells: CD56brightCD16– NK cells that are responsible for the bulk of IFNγ/TNFα production, and the more cytolytic CD56dimCD16+NK population.
More recently, we have examined differences in NK cell phenotype and function in patients with bladder cancer (BlCa) with the goal of designing immunotherapies that can overcome the suppressive milieu of the tumor microenvironment (TME)4. BlCa is the 6thmost commonly diagnosed cancer in the United States, as well as having the 6thhighest mortality. Clinically, BlCa is divided into early, non-muscle-invasive tumors (NMI), and more advanced muscle-invasive (MI) tumors5(Figure 2), and a subset of patients with metastatic and chemo-ineligible BlCa are among those for whom T cell-centric ICB has shown benefit. Using flow cytometry (FACS), and mass cytometry (CyTOF), we found that bladder tumors were well-infiltrated by NK cells, which represented the second most frequent immune lineage after CD4+and CD8+T cells. However, MI tumors had significantly fewer NK cells than NMI tumors, suggesting a role for NK cells in controlling tumor progression. We found that both NK and T cells significantly up-regulate the inhibitory receptors Tim-3 and TIGIT in both the peripheral blood and in primary tumor tissue. This over-expression occurs in both major subsets of human NK cells: CD56brightCD16– NK cells that are responsible for the bulk of IFNγ/TNFα production, and the more cytolytic CD56dimCD16+NK population.
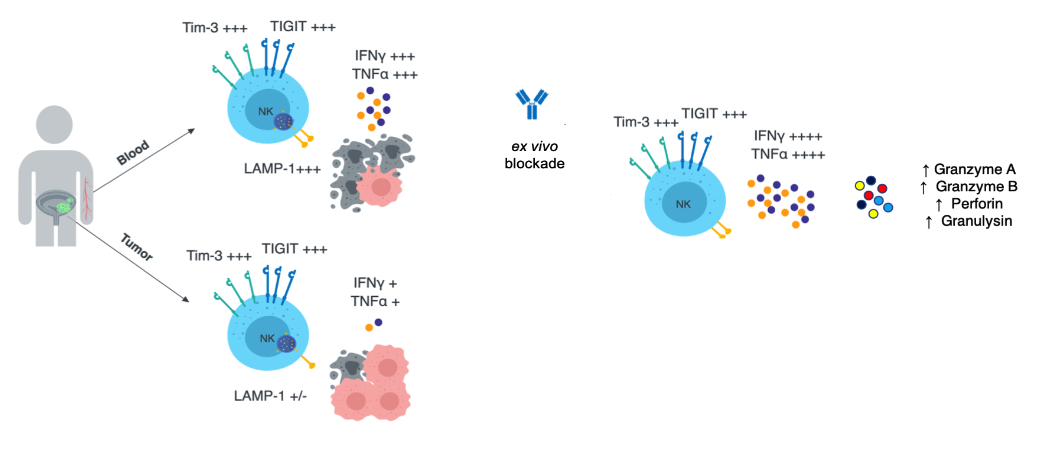
Interestingly, the magnitude of Tim-3 expression by NK cells in both tissues is tuned to the invasiveness of the primary tumor, while TIGIT is equivalently upregulated independently of tumor stage. These data suggest that pathology at a distal site may be systemically imprinted on NK cells, and that Tim-3 is a barometer of the relative severity of this process. Furthermore, Tim-3 and TIGIT expression are negatively correlated with the anti-tumor functionality of NK cells in the peripheral blood, while tumor-resident NK cells are uniformly dysfunctional, highlighting unique suppressive axes in the TME that must be overcome. As we found in patients with melanoma, ex vivo blockade of Tim-3 significantly enhances IFNy/TNFa production, but additionally improves the release of cytolytic molecules such as granzyme B and perforin (Figure 3). Importantly, this benefit was observed for the NK cells of patients with high grade, muscle-invasive disease, as well as those with less advanced tumors, and it enhanced T cell as well as NK cell function. We are also investigating the role of tumor architecture in mediating NK cell suppression to identify rational, pre-clinical interventions that may prolong immuno-surveillance at the tumor site. Ongoing work is focused on identifying conserved patterns of gene expression that mediate the unique dysfunctional phenotypes observed in the peripheral blood and tumor using single-cell RNA sequencing (scRNASeq). In parallel we are validating unique combinations of ICB that enhance the anti-tumor function of both NK and T cells in the TME.
References
- Barry, K. C.et al.A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nature medicine24, 1178-1191, doi:10.1038/s41591-018-0085-8 (2018)
- Bottcher, J. P.et al.NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell, doi:10.1016/j.cell.2018.01.004 (2018)
- da Silva, I. P.et al.in Cancer Immunol ResVol. 2 410-422 (2014).
- Audenet, F.et al.Immune phenotype of peripheral blood mononuclear cells in patients with high-risk non-muscle invasive bladder cancer. World J Urol, doi:10.1007/s00345-018-2359-7 (2018).
- Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer15, 25-41, doi:10.1038/nrc3817 (2015).
- Bhardwaj N, Farkas AM, Gul Z, Sfakianos JP. Harnessing Natural Killer Cell Function for Genitourinary Cancers, Urologic Clinics of North America (2020).
