Sleep is essential to maintain our physical and mental health; however, our lifestyle choices often promote excessive social demands at the expense of sufficient sleep. Longstanding clinical evidence demonstrates a detrimental impact of insufficient or poor-quality sleep on multiple pathologies including cardiovascular disease.
Our laboratory has recently shown that sleep fragmentation compromises the hypothalamic control over medullary hematopoiesis resulting in heightened hematopoietic stem cell proliferation and overt monocytosis and neutrophilia that exacerbate atherosclerosis. However, it remains elusive how appropriate sleep hygiene modulates adaptive immune responses and whether this affects disease pathology. This is of particular interest in light of the bidirectional relationship between sleep disturbances and the development of autoimmune disorders, such as systemic lupus erythematosus, a disease that is characterized by B lymphocyte dysfunction and confers a ten-fold risk for the incidence of cardiovascular events.
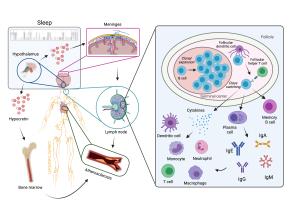
Using state-of-the-art multidisciplinary approaches for manipulating and monitoring sleep, sleep-related neuronal circuits and sleep-governed metabolic clearance pathways, we are currently investigating whether persistent sleep fragmentation impacts adaptive immunity, with special focus on B lymphocyte diversity, specificity and function. Subsequently, we aim to test how potential sleep-related changes in B cell signaling pathways as well as antibody-dependent and -independent B cell functions modulate the progression of atherosclerosis. Our ultimate goal is to delineate a sleep-instructed B cell receptor repertoire and antibody profile that may allow us to identify novel molecular targets for immunomodulatory therapeutic strategies against autoimmune disorders and cardiovascular disease.
