Canonical and noncanonical Wnt signaling
Our early studies assessed molecular processes underlying the formation of the dorsal signaling center, also known as the Spemann organizer. This analysis revealed an essential role for secreted Wnt proteins in vertebrate axis determination. Our group continued to study Wnt pathways for a number of years and obtained valuable insights related to several Wnt signaling components, from secreted Wnt antagonists and Frizzled receptors to transcription factors of the TCF family (Sokol et al., 1991; Sokol, 1996; Itoh et al., 2005; Hikasa et al, 2010). Our group also uncovered an important function of noncanonical Wnt signaling in regulating convergent extension movements during vertebrate gastrulation and neurulation (Sokol, 1996, 2000). This discovery served as a basis for subsequent molecular characterization of the Wnt/PCP pathway. More recently, we linked Wnt/Planar cell polarity (PCP) signaling to apical constriction, another fundamental morphogenetic event that leads to embryonic tissue folding (Ossipova et al., 2014, 2015).
The establishment of apicobasal and planar cell polarity in embryonic ectoderm
We have shown the involvement of apical-basal and planar cell polarity proteins in cell fate determination during epidermal differentiation, neurogenesis and neural crest development, using mouse, human and Xenopus progenitor models (Dollar et al., 2005; Ossipova et al., 2009; Lake and Sokol, 2009). We have recently shown that Wnt proteins can direct PCP in the vertebrate neural plate (Chu and Sokol, 2016). These studies provide insights into asymmetric neural progenitor divisions during neurogenesis, multiciliated cell differentiation in the epidermis and mechanistically connect PAR, Notch and Wnt signaling to PCP.
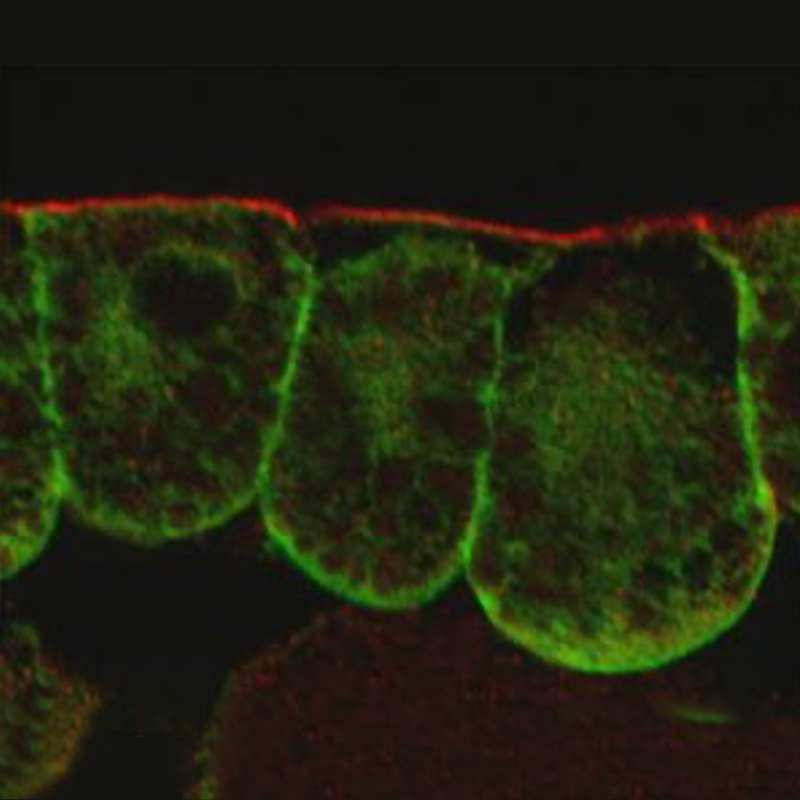
Planar cell polarity in neural tube closure
 The conserved core PCP proteins (Vang/Vang-like, Prickle/Pk, Dishevelled/Dvl, Frizzled/Fz and Flamingo/Celsr) have been discovered in Drosophila genetic studies. In vertebrates, core PCP protein complexes accumulate at opposite cell edges along the anteroposterior body axis, marking axial polarity. The significance of the core PCP proteins extends far beyond being polarity markers, as their vertebrate homologs function in key developmental processes, including gastrulation movements, neural tube closure, branching morphogenesis, formation of functional cilia and left-right patterning. How PCP proteins coordinate collective cell behaviors remains a challenging mystery that has been occupying scientists for almost three decades.
The conserved core PCP proteins (Vang/Vang-like, Prickle/Pk, Dishevelled/Dvl, Frizzled/Fz and Flamingo/Celsr) have been discovered in Drosophila genetic studies. In vertebrates, core PCP protein complexes accumulate at opposite cell edges along the anteroposterior body axis, marking axial polarity. The significance of the core PCP proteins extends far beyond being polarity markers, as their vertebrate homologs function in key developmental processes, including gastrulation movements, neural tube closure, branching morphogenesis, formation of functional cilia and left-right patterning. How PCP proteins coordinate collective cell behaviors remains a challenging mystery that has been occupying scientists for almost three decades.
We have established a new model for neural plate PCP along the anteroposterior axis and found that this polarity requires both Wnt and Myosin II/ROCK signaling. We have also characterized PCP along the mediolateral axis that is likely involved in the regulation of apical constriction in the neural plate. Our results also suggest the involvement of PCP proteins in radial cell intercalations (Ossipova et al., 2014, 2015; Sokol, 2016).
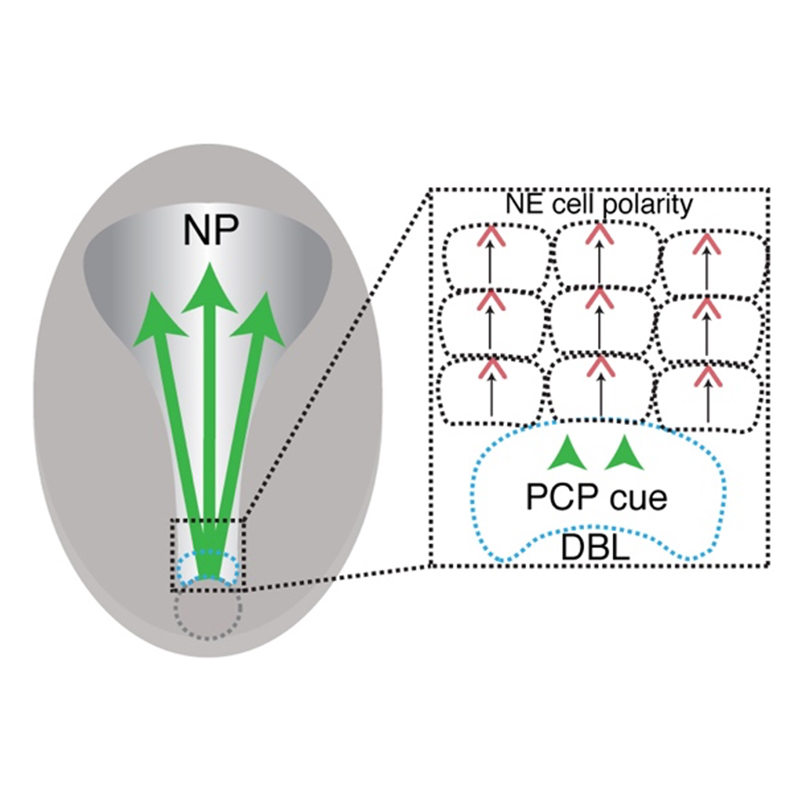
We have shown that Wnt proteins can serve as cues for PCP and are required for neuroectodermal PCP (Chu and Sokol, 2016). In a live imaging study, we observed that fluorescently labeled Pk3 initially polarized in the posterior region of the neural plate (NP) and later polarized in the anterior NP (Mancini et al., 2021). A surgical incision in the middle of the NP disrupting neuroectoderm (but not underlying mesendoderm) prevented the establishment of anterior NP PCP. These findings indicate that PCP is induced by a signal spreading from the posterior embryonic region. Supporting this conclusion, transplanted blastopore lip caused PCP reversals in the anterior NP (Mancini et al., 2021). These observations demonstrate that a cue from the dorsal lip is both necessary and sufficient for PCP induction.
We discovered feedback regulation between core PCP proteins that leads to their segregation to opposite sides of the cell. Using in vivo proximity biotinylation, we found that the apical polarity protein Par3 is planar polarized in the NP and promotes the formation of the anterior Vangl2-Pk3 complex by recruiting Pk3 to the apical surface (Chuykin et al., 2018). With this technique, we also demonstrated a negative role of Fz3 in the formation of the Vangl2-Pk3 complex (Chuykin et al., 2021). This regulatory feedback contributes to the anterior restriction of the PCP complex in neuroepithelial cells.
Our recent work 1) has shown that PCP is induced by localized embryonic signals (Mancini et al., 2021), 2) demonstrated a new feedback regulation between Fz3 and Vangl2 (Chuykin et al., 2021), 3) identified Wtip, Shroom3 and LMO7 as candidate molecules that link core PCP complexes to actomyosin contractions (Chu et al., 2018, Matsuda et al., 2022). We continue to investigate a) how cells polarize in response to a cue, b) how the cell polarity translates into spatially restricted activation of Myosin II, and c) how actomyosin contractions alter cell shape and coordinate collective cell movements.
How does cell polarity control actomyosin contractions and morphogenesis ?
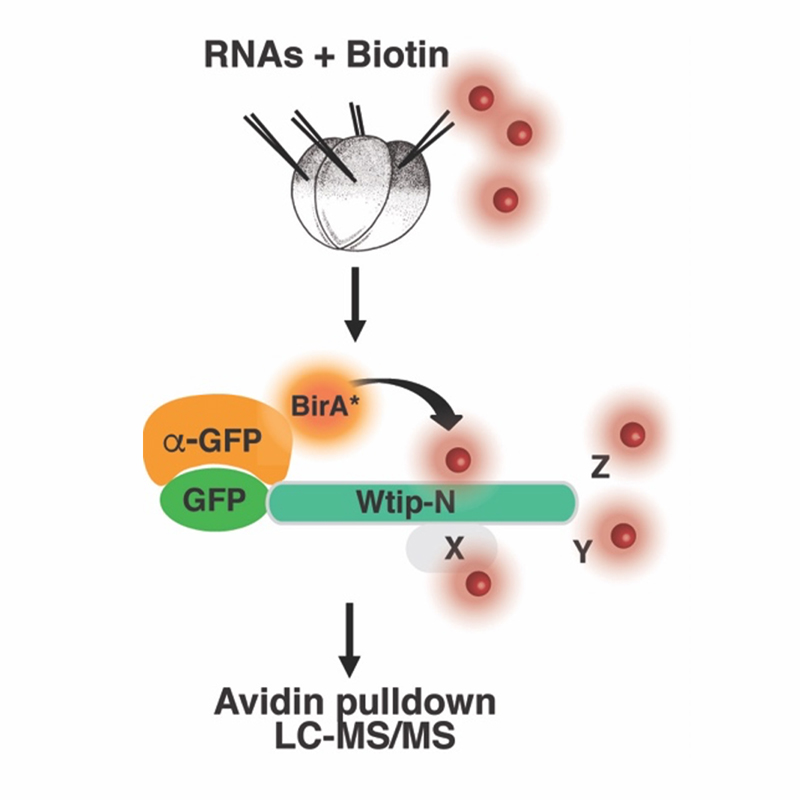
Based on published literature and our observations, we hypothesize that PCP signaling locally modulates actomyosin contractility to produce anisotropic forces needed for morphogenesis. Once a tissue becomes polarized, core PCP complexes may engage downstream effectors that are necessary to change cell shape leading to NP folding (Nishimura et al., 2012; Ossipova et al., 2014; Sokol, 2016). During the past funding period, we focused on relatively understudied Prickle proteins and identified molecules that link Pk3 to cell junctions, including the apical determinant Par3 (Chuykin et al., 2018) and the LIM domain protein Wtip (Chu et al., 2016). Wtip belongs to the Zyxin/Ajuba family implicated in actomyosin dynamics and mechanotransduction (Chu et al., 2018; Ibar et al., 2018; Rauskolb et al., 2014). We developed a proximity biotinylation approach based on anti-GFP single domain antibody (sdAb)-mediated targeting of BirA and ideantified the centrosomal maturation factor SSX2IP/ADIP as a Wtip-binding protein (Reis et al., 2021).
How does cell polarity control actomyosin contractions and morphogenesis ?
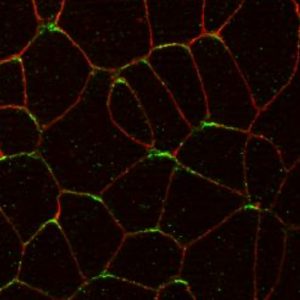
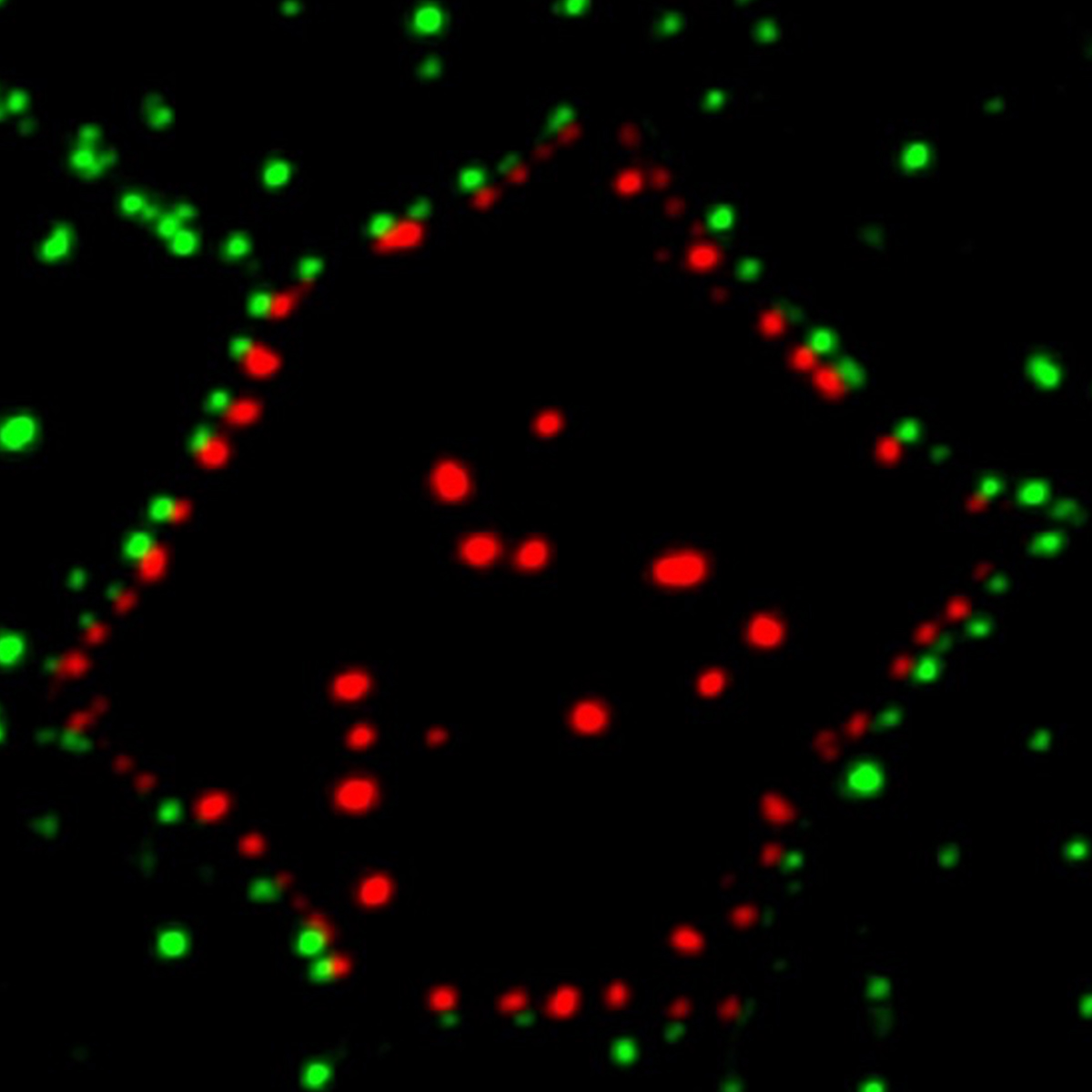
To modulate cell shape, contractile actomyosin complexes need to localize at cell junctions. We recently discovered that the localization of the LIM domain protein Wip to intercellular junctions likely depends on a mechanical force (Chu et al. 2018). Wtip, a Prickle3-interacting LIM domain protein, can sense Myosin II activity and intercellular tension (Chu et al., 2018). Moreover, Wtip forms cytoplasmic aggregates or puncta opposing each other across the cell membrane (see image), supporting the idea that neighboring cells mechanically communicate with each other.
Our recent studies have focused on LMO7 protein that is also sensitive to mechanical force. A mutation in the fly homologue of LMO7, smallish, has morphogenesis defects in Drosophila embryonic epidermis (Beati et al., 2018). Importantly, LMO7 triggers apical constriction in Xenopus ectoderm (Matsuda et al., 2022). Of note, LMO7 is distributed in ectoderm as a double line adjacent to the apical junction, mimicking the distribution of Myosin II heavy chain. We hypothesize that LMO7 regulates the organization of Myosin II and have shown that LMO7 recruits Myosin II heavy chain to the cell cortex (Matsuda et al., 2022). Our model is that planar polarity and force balance are responsible for neural plate folding in the mediolateral axis but not in the anteroposterior axis.
Sergei Y Sokol, PhD
sergei.sokol@mssm.edu
Annenberg Building Floor 25th Floor Room 86A
1468 Madison Ave
New York, NY 10029
Tel: 212-241-1757
Fax: 212-860-9279
