by PAIREDProject | Dec 7, 2023 | Autonomics, Immune
Last month we were excited to have a new manuscript published online in the Journal of Neuroimmune Pharmacology exploring how dysfunction of the autonomic nervous system (ANS) might impact immune function. It’s been known for a long time that the sympathetic nervous system sends nerve fibers into all the organs of the immune system. This includes so-called “primary lymph organs” like the thymus and the bone marrow where immune cells are born, and “secondary lymph organs” like the lymph nodes and spleen where immune cells congregate. It’s also known that immune cells have specialized receptors for norepinephrine, the neurotransmitter released by sympathetic nerve fibers, and that immune cells change their behavior in response to norepinephrine. But hardly any of this research has been done in humans, probably because its difficult (if not impossible) to directly image or measure the activity of these tiny nerves deep inside the human body.
At the PAIRED Project we use a battery of autonomic function tests (check out a video of alum, Alyha Benitez, demonstrating) to approximate the function of the ANS and rate it on a simple 0-10 scale called the Composite Autonomic Severity Score (CASS). For this new study, we divided research participants into four groups based on their CASS score. We then measured a panel of 41 cytokines in their blood. These cytokines are substances produced by immune cells that have complex effects on the body. Ideally, they help coordinate the fight against infection, but when not properly regulated they can cause damage through excessive inflammation. The figure below is the main finding from the study which shows four different cytokine networks, one for each of the four groups of participants.
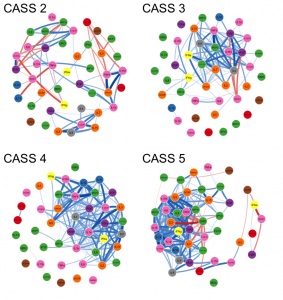
In the network each circle represents one cytokine, and a blue connector between the circles indicates a positive correlation. Positive correlation means they tend to do the same thing, like if the level of one cytokine is high in a participant then so is the level of the connected cytokine. The differences between the networks is visually striking: the networks with higher CASS scores are much bluer, much more strongly connected. This means that in the higher CASS groups (i.e., the people with worse autonomic function) patients’ cytokines tend to exhibit an “all or nothing” response. In a kind of “mob mentality,” if one cytokine is low, they all tend to be low, and if one cytokine is high, they all tend to be high.
Why might this be? Perhaps a normal ANS, with its nerve fibers reaching into the places where immune cells reside, instructs those cells on what cytokines to release, allowing for an appropriately restrained immune response when a threat is detected. If that’s true then loss of these nerve fibers might result in an overzealous less-targeted response to threat. A normal ANS might also be responsible for assessing the threat level in the first place through its sensory branches, and so an immune system without this neurologic control system may be both blind and bellicose.
These are still early findings, and a lot of work remains. This study examined cytokines, which are released from immune cells, but we didn’t examine the immune cells themselves. We also used the CASS as a summary score of autonomic function but we still don’t have a good way of specifically measuring the nerve fibers innervating lymph nodes and other immune organs. These are puzzles still to be worked out as we seek to expand our understanding of neurologic control of immunity.

by PAIREDProject | Jun 23, 2023 | Autonomics, Immune
This month our manuscript “Autonomic neuropathy as a predictor of morbidity and mortality in people living with HIV: a retrospective, longitudinal cohort study” was published in Neurology Clinical Practice. The title kind of says it all, we found that autonomic neuropathy seems to be making people with HIV sick. But what is autonomic neuropathy, why are people with HIV getting it, and how is it leading to poor health outcomes?
The story starts over ten years ago. Back then, as our team was first establishing a research program in NeuroHIV, we were reading and learning as much as we could about how HIV damaged peripheral nerves leading to sensory neuropathy and its symptoms of numbness, tingling and pain in the feet. We were especially fascinated by the work of Dr. Ahmet Hoke’s lab at Johns Hopkins which showed that an HIV protein, called gp-120, generated an inflammatory response that was toxic to nerves, and that the energy-producing mitochondria were also damaged in HIV-associated sensory neuropathy. If sensory nerves were under this multipronged attack, what was happening to autonomic nerves? Autonomic nerves are very similar structurally to sensory nerves, but since they control the function of internal organs, their malfunction can cause symptoms that often go undiagnosed since they don’t sound neurologic: racing pulse, dizziness, nausea, urinary and sexual dysfunction. And it’s not just symptoms to worry about, particularly in the heart, autonomic neuropathy can have very serious consequences. People with autonomic neuropathy due to diabetes have an increased risk of arrhythmias and sudden cardiac death.
We wanted to know if autonomic neuropathy was affecting people with HIV, so we designed a simple study. We recruited a “waiting room” sample from our primary care HIV clinic. Basically anyone who wanted to participate and could do so safely was included. We did tests for autonomic and sensory neuropathy and administered questionnaires to see if patients were experiencing symptoms. We made three important discoveries:
- Autonomic neuropathy was common overall, but especially in participants with sensory neuropathy in the feet.
- Lots of patients had the symptoms listed in the autonomic symptom questionnaire, regardless of whether or not they had autonomic neuropathy, so the symptoms weren’t helpful in establishing the diagnosis.
- Participants with autonomic neuropathy were more likely to have other medical conditions in addition to HIV.
The last finding interested us especially, because it likely meant one of two things. One possibility was that the co-existing medical conditions were an additional strain on the autonomic nerves which increased the likelihood of autonomic neuropathy. However, an alternative possibility was that the autonomic neuropathy was causing other medical conditions in people living with HIV by disrupting normal organ function. This latter idea was intriguing but wasn’t provable. At that time we only had data from a single time point, with no way of knowing whether autonomic neuropathy was the chicken or the egg. Assessing causality would take time. You’d have to wait and see if patients with autonomic neuropathy ended up with worse health outcomes than could be explained by their other medical illnesses alone. If so, it would suggest that the increased risk could be attributed to autonomic neuropathy.
So we waited, almost 10 years, and then we looked into our electronic health record to see how our study participants had been doing all that time. Fortunately, most were alive and well, many still seeing the same doctors. But not all. Some had left the health system and others had experienced deteriorating health. There are lots of ways to quantify overall health; we chose an outcome based on the landmark SMART study, which led to the current practice of using antiretroviral therapy continuously without treatment interruptions. The outcome (which continuous antiretroviral therapy helped to avoid in SMART) was the occurrence of any of the following: 1) death from any cause; 2) major cardiovascular event; 3) major cerebrovascular event; 4) major new kidney disease; 5) major new liver disease. We liked that this composite outcome was broad enough to capture a potential effect of autonomic neuropathy on multiple different organ systems.
Here is the main figure from our publication which came out this month in Neurology Clinical Practice:

It shows that even after accounting for baseline health status, people living with HIV and autonomic neuropathy (shown in the lighter grey) had about three times the risk of experiencing one of the poor health outcomes. This suggests that autonomic neuropathy may be a cause of significant organ dysfunction in many people living with HIV. This is clearly worrisome, and in order to develop strategies to prevent or reverse autonomic neuropathy and its adverse impact on the body, we must first understand the underlying mechanisms. This will be challenging but essential for fulfilling our PAIRED Project mission: “to make the best use of our unique skills as neurologists and neuroscience researchers to improve the health of the members of our surrounding communities, and to make the discoveries that will lead to even better health outcomes in the future.”





Recent Comments