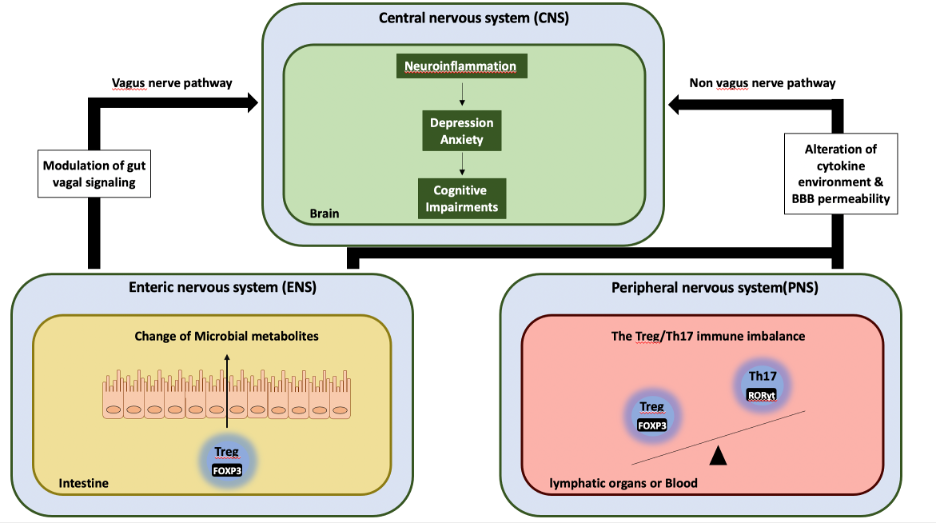
Among the various causative factors, depression has emerged as a critical factor, increasing the risk of developing Alzheimer’s disease (AD) by 2 to 4-fold. Thus, mitigating depression-like behavior at early-life could lessen the risk of AD at later stages; and that elucidating underlying molecular mechanisms linking depression to AD would offer feasible target for preventing AD progression. Forkhead box P3 (FOXP3) is a transcription factor modulating the function of regulatory T cells (Tregs) in peripheral immune system, in inducing depression-like behavior in mice following chronic unpredictable stress. However, it is not known if alleviating depression by modulating FOXP3 could reduce the risk of developing AD-like pathologies.
In studies led by Dr. Yang, we aim to modulate FOXP3 functions using diverse AD model platform:
1) Mouse model of AD To investigate underlying AD-related mechanisms, we use several mouse models including FOXP3, 5XFAD, and a double transgenic mouse model (5xFAD x FOXP3).
2) Crosstalk between peripheral immune system and brain in AD Blood immune cells can affect brain functions through several pathways such as blood brain barrier (BBB), glymphatic pathway and blood CSF barrier. We are investigating whether reduction of peripheral FOXP3 in AD model mice alters blood brain barrier (BBB) permeability leading to neuroinflammation, and thereby exacerbating brain cognitive behaviors.
3) Gut brain axis in AD The gut-brain axis (GBA) is now considered as a promising therapeutic target for AD. We focus on the molecular mechanisms caused by the deletion of FOXP3 in gut to find out cross-talk between the gut microbiota and the GBA in AD.