Our work concentrates on the mechanisms for the onset of asthma. One large research focus involves establishing and studying a birth cohort from Northern Manhattan (Columbia Center for Children’s Environmental Health), determining the importance of environmental allergens, traffic-related pollutants, and phthalate exposure to the onset of allergies, asthma, and allergic immune responses. A major emphasis is on the role of prenatal and early postnatal exposure on later pediatric and adolescent asthma risk. Additional areas of research include identifying novel genetic by environment interactions and epigenetic by environment interactions important to the onset of asthma.
Children’s Respiratory Research Workgroup (CREW) consortium:
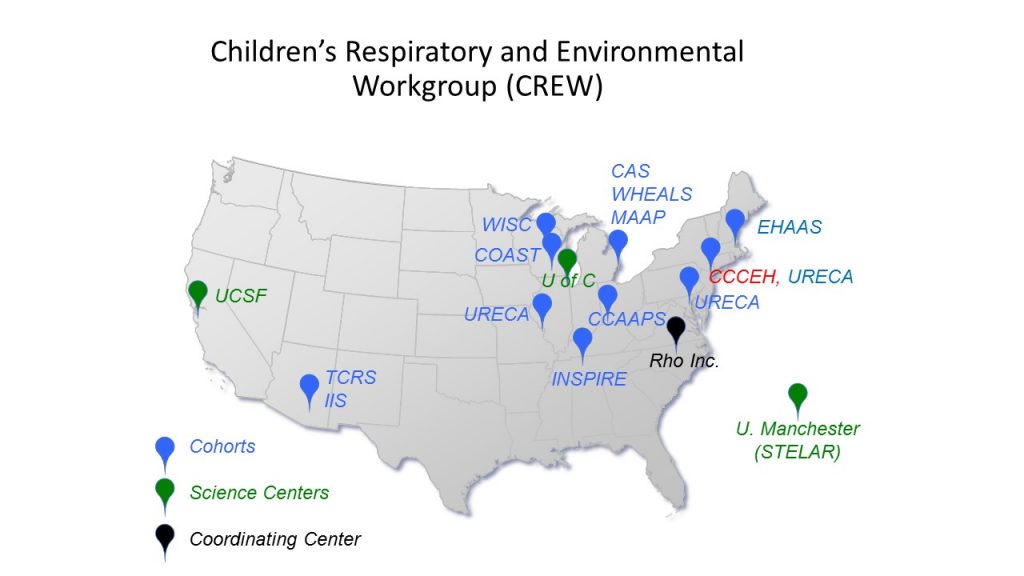
We have teamed up with investigators that lead 12 asthma birth cohorts across the U.S. to establish the Children’s Respiratory Research Workgroup (CREW) consortium. CREW proposes to identify specific types of childhood asthma, develop an understanding of what early life environmental influences cause these different types of asthma and when, and identify targets for future efforts aimed at preventing childhood asthma. CREW will include data from a large number of children (over 9,000 at birth, 6,000-7,000 who are still being followed, and at least 5,667 expected to enroll in CREW) and their families, with broad diversity in terms of ethnicity, family characteristics, neighborhoods and geographic locations. One of the primary goals of CREW is to put together sets of data and samples of participating cohorts to identify phenotypes of childhood asthma (i.e. specific subtypes of asthma that can be distinguished by clinical features such as natural history, triggers, exacerbation frequency, concurrent allergies, lung function, sex, etc). As we obtain mechanistic insights about personal and early life risk factors, we will connect asthma phenotypes with underlying causes and pathogenic mechanisms to define endotypes of childhood asthma.
CREW is itself a “cohort” funded as part of the NIH Environmental Influences on Child Health Outcomes (ECHO) program. As such, in addition to sharing data and samples among CREW investigators, CREW cohort data will also be shared with the larger ECHO program.
Mitochondrial DNA biomarkers to assess responses to changes in personal environmental exposures in pediatric urban asthma:
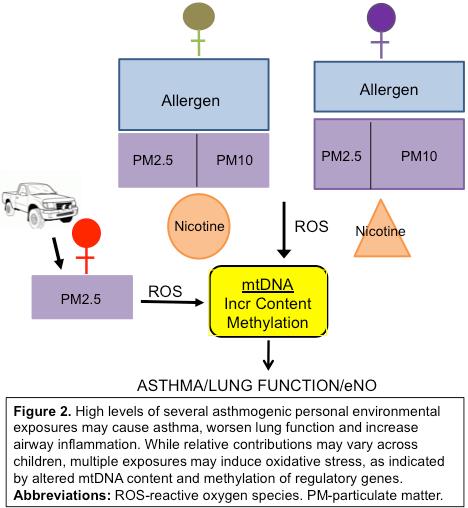
We are interrogating novel biomarkers that capture responses to multiple environmental stressors, and are highly sensitive to the dynamics of dysregulation, on lung function and airway inflammation. Mitochondrial (mt) DNA lacks protective histones and possesses diminished DNA repair mechanisms, rendering it more susceptible than the nuclear genome to damage by reactive oxygen species (ROS), interrupting redox homeostasis, and causing inflammation. Our group and others have shown that exposure to air pollution and allergens increases intracellular levels of ROS in mitochondria. The ensuing mtDNA damage increases its quantity or content. Urban exposures also may alter methylation at key mitochondrial replication and transcriptional control regions. Asthmatics may be more susceptible. Mitochondrial DNA damage can reverse, possibly more readily than other plasma biomarkers of oxidative stress. Our objective is to elucidate the dynamic interplay between reductions in multiple inflammatory urban exposures, attributable changes in mtDNA content and methylation, and improvements in pediatric asthma outcomes over time. We intend to capture the pivotal role of novel mt biomarkers in measuring the dynamic biological responses following induction and remediation of oxidative damage, triggered by a child’s changing personal environment. Results could direct more effective personalized biomarker-guided therapy or intervention, including environmental remediation, pathway inhibitors, or dietary interventions, for children with persistent asthma, and for all children exposed to an urban environment.
Air pollution exposure and complex diseases-mouse models:
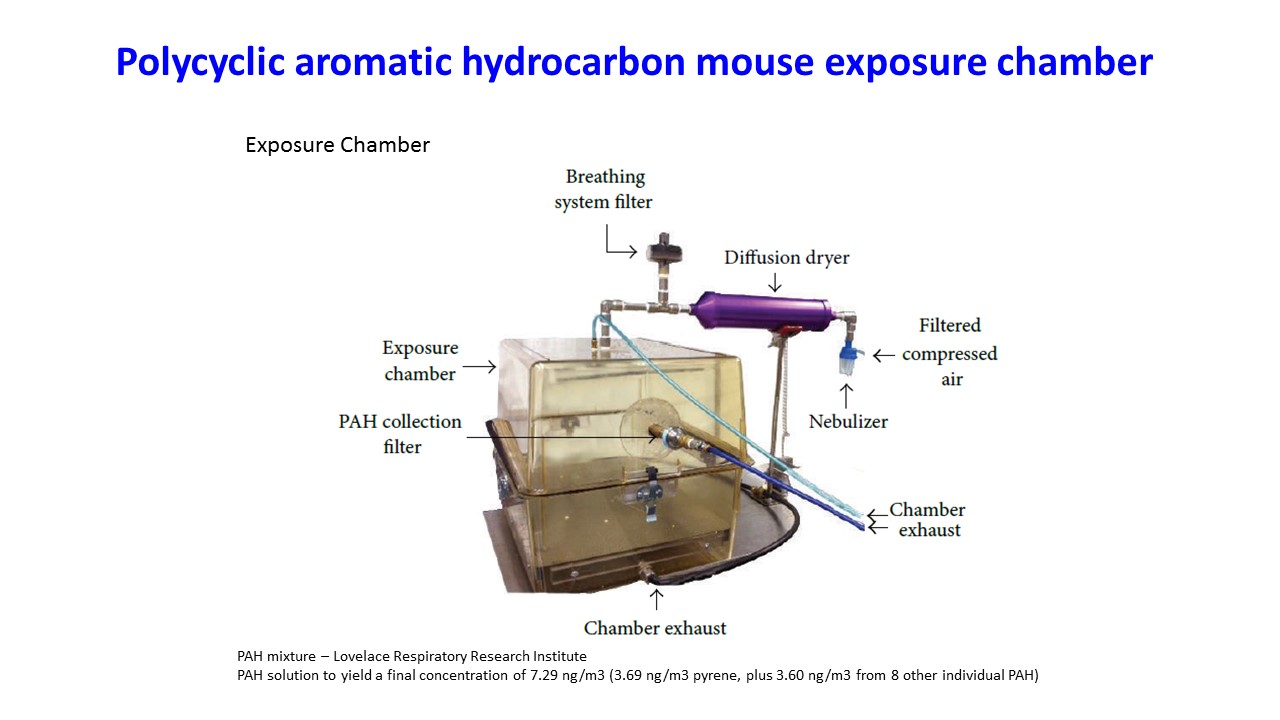
We also have established several mouse models examining the importance of prenatal and postnatal environmental exposures on risk for asthma and other complex diseases that include obesity and neurocognitive dysfunction. More recent research focuses on the mechanistic effects of prenatal and pregnancy-associated polycyclic aromatic hydrocarbon exposure on breast cancer risk in mice.
Endocrine Disruption, Hormones, and Sex Differences in Adolescent Airway Health
