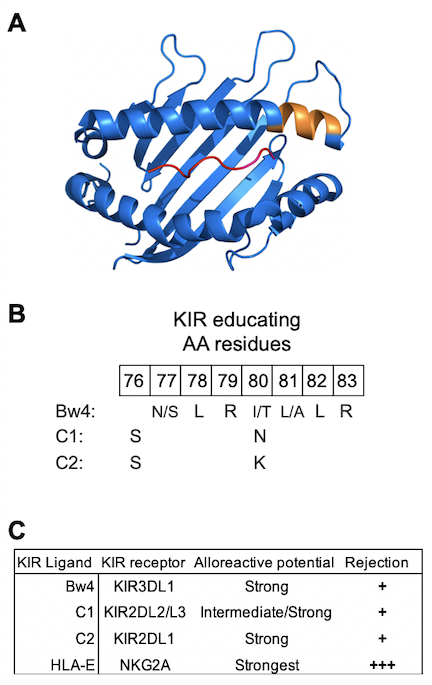
 Natural Killer (NK) cells are a major lineage of human lymphocytes with vital functions in innate and adaptive immunity. These functions are mediated through a process of ‘education’ defined as the training of NK cells to distinguish diseased or allogeneic cells, which exhibit perturbed expression of HLA class I, from healthy, autologous cells having normal expression of HLA class I. Education is achieved through diverse interactions of highly polymorphic HLA-A, -B and -C molecules with equally polymorphic killer immunoglobulin-like receptors (KIR). They are complemented by the conserved interactions of HLA-E with CD94/NKG2A (NKG2A) heterodimers. A subset of HLA-A and -B and all HLA-C genes encode conserved epitopes expressed between residues 76-83 that are recognized by KIR receptors (Fig. 1A and 1B). Polymorphisms in KIR genes result in differences in binding avidities and specificity for their respective inhibitory ligands and may predict different degrees of alloreactivity (Fig. 1C). Because the KIR genes on chromosome 19 segregate independently of the HLA class I genes on chromosome 6, a vast diversity of compound genotypes (KIR plus HLA class I) are present in human populations. Phenotypically, this produces functionally distinct NK cell repertoires, a natural variation that has profound effects on susceptibility to infection and on alloreactivity influencing the outcome of hematopoietic cell transplantation (HCT). Although the system is intrinsically complicated, it is built up in layers according to simple and basic principles. Little is known about the role of NK cells in graft tolerance vs graft rejection for solid organ transplantation, although impaired NK cell functions in kidney transplant recipients associated with two-fold increased risk for combined endpoints of metastatic cancer, cancer-related death or septic death.
Natural Killer (NK) cells are a major lineage of human lymphocytes with vital functions in innate and adaptive immunity. These functions are mediated through a process of ‘education’ defined as the training of NK cells to distinguish diseased or allogeneic cells, which exhibit perturbed expression of HLA class I, from healthy, autologous cells having normal expression of HLA class I. Education is achieved through diverse interactions of highly polymorphic HLA-A, -B and -C molecules with equally polymorphic killer immunoglobulin-like receptors (KIR). They are complemented by the conserved interactions of HLA-E with CD94/NKG2A (NKG2A) heterodimers. A subset of HLA-A and -B and all HLA-C genes encode conserved epitopes expressed between residues 76-83 that are recognized by KIR receptors (Fig. 1A and 1B). Polymorphisms in KIR genes result in differences in binding avidities and specificity for their respective inhibitory ligands and may predict different degrees of alloreactivity (Fig. 1C). Because the KIR genes on chromosome 19 segregate independently of the HLA class I genes on chromosome 6, a vast diversity of compound genotypes (KIR plus HLA class I) are present in human populations. Phenotypically, this produces functionally distinct NK cell repertoires, a natural variation that has profound effects on susceptibility to infection and on alloreactivity influencing the outcome of hematopoietic cell transplantation (HCT). Although the system is intrinsically complicated, it is built up in layers according to simple and basic principles. Little is known about the role of NK cells in graft tolerance vs graft rejection for solid organ transplantation, although impaired NK cell functions in kidney transplant recipients associated with two-fold increased risk for combined endpoints of metastatic cancer, cancer-related death or septic death.
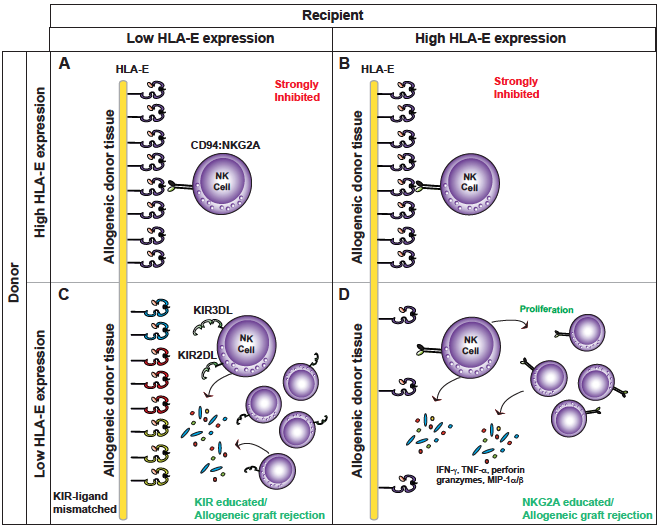
HLA-E elicits an inhibitory signal via its interactions with NKG2A on NK cells and is considered the most dominant inhibitory signal for an NK cell. HLA-E expression critically depends on availability of a highly conserved nonamer peptide (VMAPRTLLL) derived from the leader sequence of an HLA-A, -B and -C polypeptide. Analyses of genetic variation in HLA-A, -B and -C genes indicate that human populations are split into two roughly equal groups with ~40% expressing high levels of HLA-E (higher threshold for NK cell activation) and the remaining individuals expressing low levels of HLA-E. Our central hypothesis is that inhibition of NK cells mediated by HLA-E in transplant recipients will (i) determine the degree of NK cell alloreactivity to donor tissue; and (ii) provide a biomarker for kidney injury, graft rejection, and overall survival. For instance, we predict that NK cells from recipients with high levels of HLA-E receiving allogeneic transplant from donors with low HLA-E will be more alloreactive compared to donor tissue expressing equally high levels of HLA-E (Figure 2)
Cytomegalovirus (CMV) infection is one of the most common complications associated with kidney transplantation in settings of primo-infection as well as reactivation. Approximately, 40% of CMV-infected individuals have large expansions of ‘adaptive’ NK cells (range, 0-70% of total circulating NK cells) with enhanced reactivity for antibody-dependent cellular cytotoxicity (ADCC), which may prove deleterious for kidney transplantation in recipients with high levels of donor-specific allo-antibodies. Adaptive NK cells preferentially express the activating isoform of NKG2A: NKG2C, and its interaction with HLA-E elicits an activating signal. Further, 100% of adaptive NK cells express self-HLA-C-reactive inhibitory KIR2DL receptors, suggesting that adaptive NK cells are uniquely poised for triggering alloreactive or ‘non-self’ immune responses (Figure 3). Thus, we further predict that CMV infection and the presence of adaptive NK cells will differentiate risk of high HLA-E expression on graft rejection in recipients.