Maite Sanchez-Aparicio
The goal of my work is to understand the mechanisms involved in the innate immune response against viral infections. I am mainly focused on identifying and characterizing the spatio-temporal events in the RIG-I like receptor pathway that lead to the production of type I IFN, and the mechanisms underlying the inhibition of this response by viruses. I currently work on different projects involving different RNA viruses, combining techniques from microscopy, cell biology, virology and biochemistry. In particular, we have developed a Bimolecular Complementary Assay (BiFC) to characterize the interaction between viral and host cell proteins.
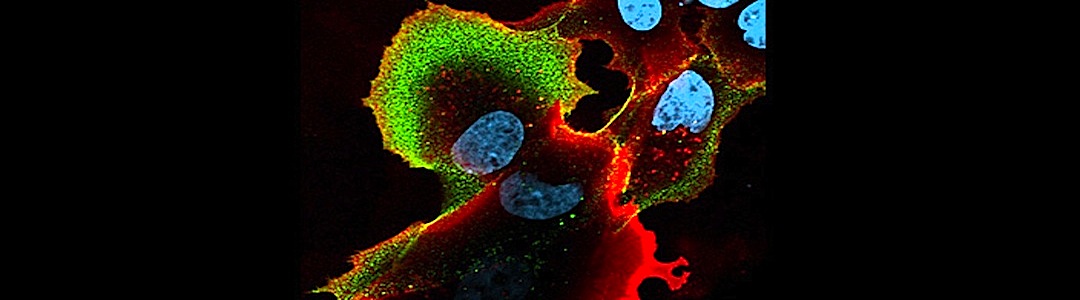
HeLa cells infected with Influenza A virus. RIG-I and MAVS co-localize with the mitochondria. Structured illumination microscopy (SIM) fluorescence. Image acquired with a 63x objective.
Danae Fonseca, Ph.D
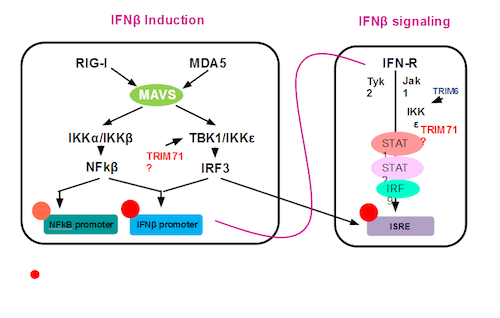
Viral infection triggers a fast and effective cellular response mediated primarily by the production of INFβ that induces an anti-viral state through complex signal cascades. Therefore, the regulation of its induction and subsequent IFNβ signaling needs to be tightly controlled. There is growing evidence implicating the members of Tripartite-motif (TRIM) protein family of E3 ligases as critical players in this regulation. However, the exact role, mechanism of action, and the physiological relevance of their activity in vivo still remain poorly understood. Previous work in our lab revealed that a large number of TRIMs play critical roles as enhancers in the regulation of innate immune signaling pathways. My research focuses on the study of TRIM71 and its role in innate immune signaling. It was previously shown that TRIM71 overexpression strongly increased the 2CARD-RIG-I-dependent activation of the INFβ and ISRE promoters. I am now investigating the specific level at which TRIM71 exerts its function, whether its E3 ubiquitin-ligase activity is important for its function, and its potential substrates in order to delineate the molecular mechanism by which it regulates responses to viral infection.
Giuseppe Pisanelli, Ph.D.
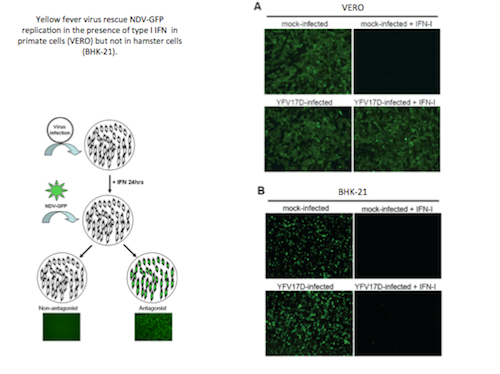
Yellow fever virus (YFV) is the etiologic agent of yellow fever. There are no antivirals available to treat yellow fever, and attempts to develop YFV-specific antivirals or to develop alternative vaccines have been hampered by the lack of an immunocompetent mouse model of yellow fever. It has been reported that both the wild-type (Asibi) strain and the 17D strain of yellow fever infect mice with defects in type I IFN (IFN-I) signaling. This suggests that the IFN-I response serves as the major barrier to mouse infection. We have recently shown that YFV antagonizes IFN-I signaling in human and non-human primate cells and that the NS5 proteins of both YFV-Asibi and YFV-17D bind to and sequester human STAT2 after interferon signaling. My research project’s aim is to identify the host restriction factors responsible for the inability of the YF-NS5 protein to bind to murine STAT2 and inhibit murine IFN-I signaling. This will be potentially useful for the development of a Yellow Fever mouse model.
Wing-Hong Kwan, Ph.D
As specialized antigen-presenting cells lining all natural barriers (skin, lung, and gut) DCs and macrophages survey the tissue and maintain its integrity. Partial or complete deficiencies in the development of DCs or macrophages impair tissue integrity in the steady state and induce maladaptive immune responses upon infection. My work focuses on the role of host factors (complement components and IFITMs) that regulate DC and macrophage responses during influenza virus infection, and how they impact T-cell responses.
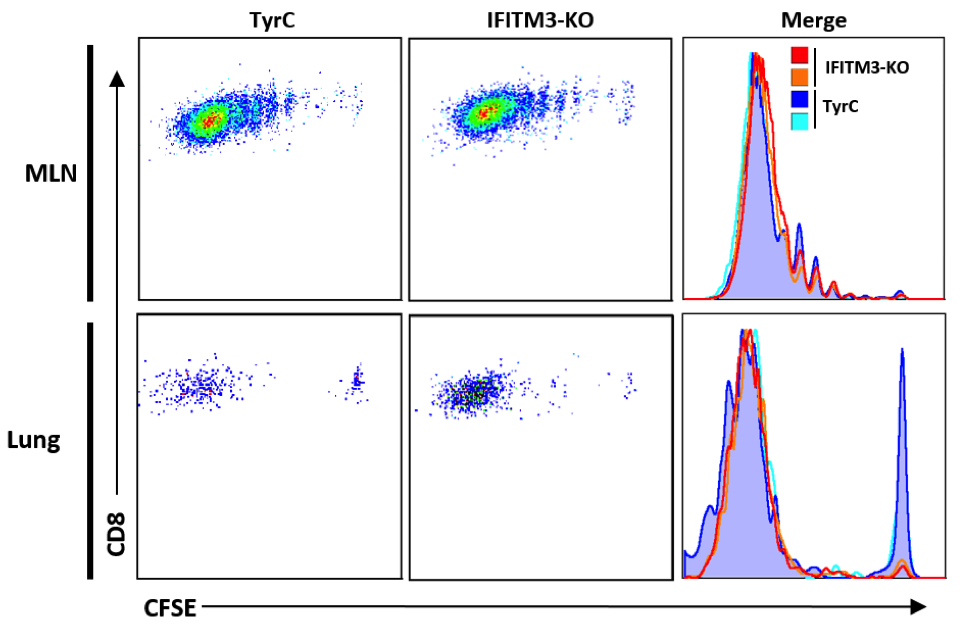
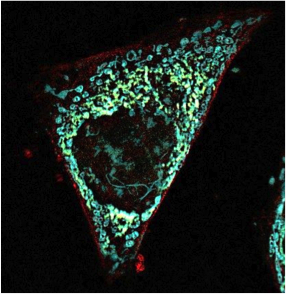
CFSE labeled OTI cells were transferred into Control or IFITM3 deficient mice infected by influenza virus expressing OVA 257-264. D=5 post-infection, lung and mediastinal lymph node were processed and OTI cell proliferation was assessed by CFSE dilution by Flow Cytometry.
Lisa Miorin, Ph.D
The prompt and tightly controlled induction of type I interferon (IFN) is a central event of the immune defense against viral infection. Recently, we have shown that several members of the tripartite motif-containing (TRIM) protein family positively regulate the host cell immune response. However, the mechanism of action of most of these factors and the physiological relevance of their activity in vivo still remain poorly investigated. My work in the García-Sastre laboratory focuses on understanding the molecular mechanisms through which novel TRIM proteins modulate innate immune signaling pathways and viral restriction using in vitro biochemical approaches as well as developing relevant small animal models for in vivo studies.