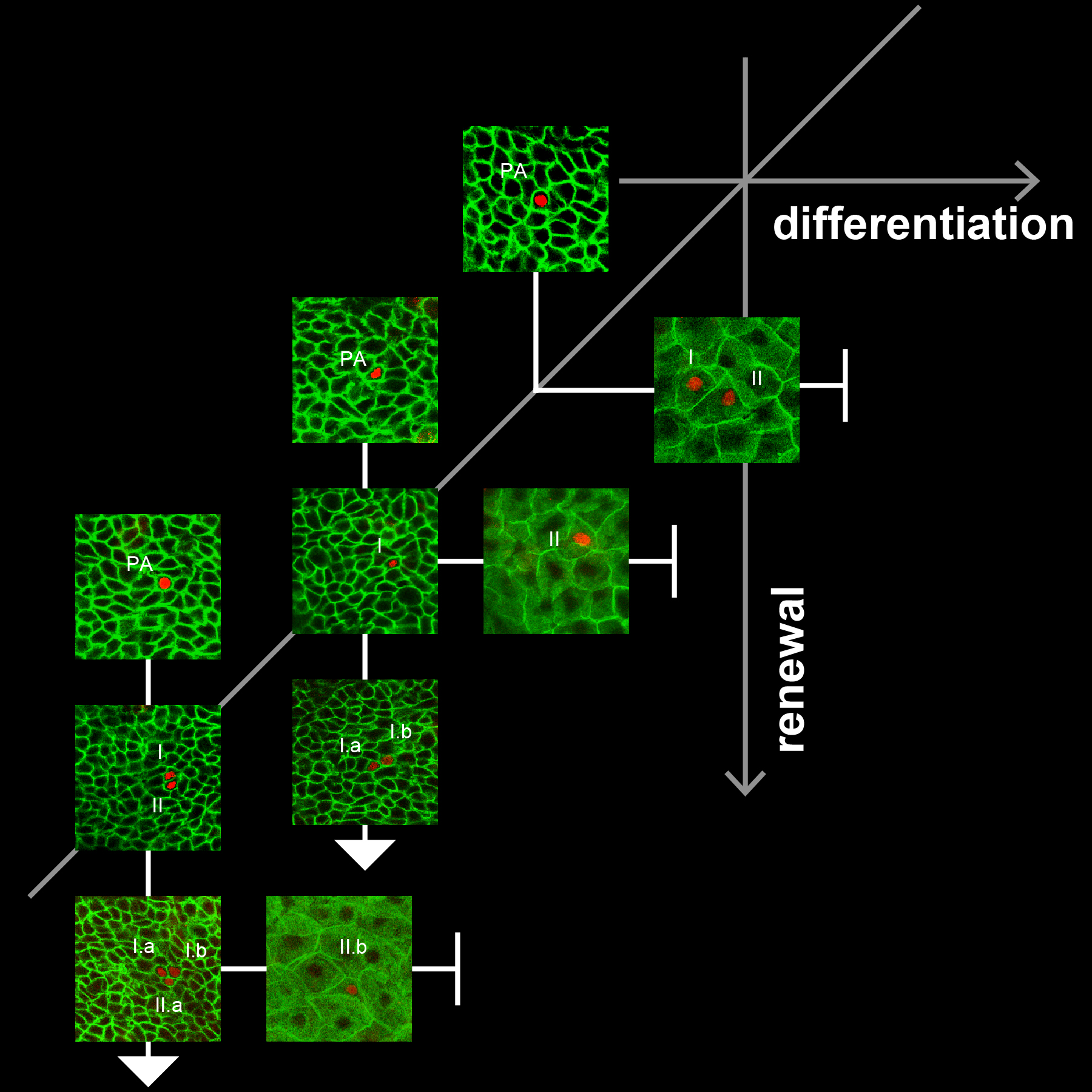
Our lab studies the causal relationships between cancer lesions, tissue damaging stresses, environmental signals and epithelial clonal dynamics.
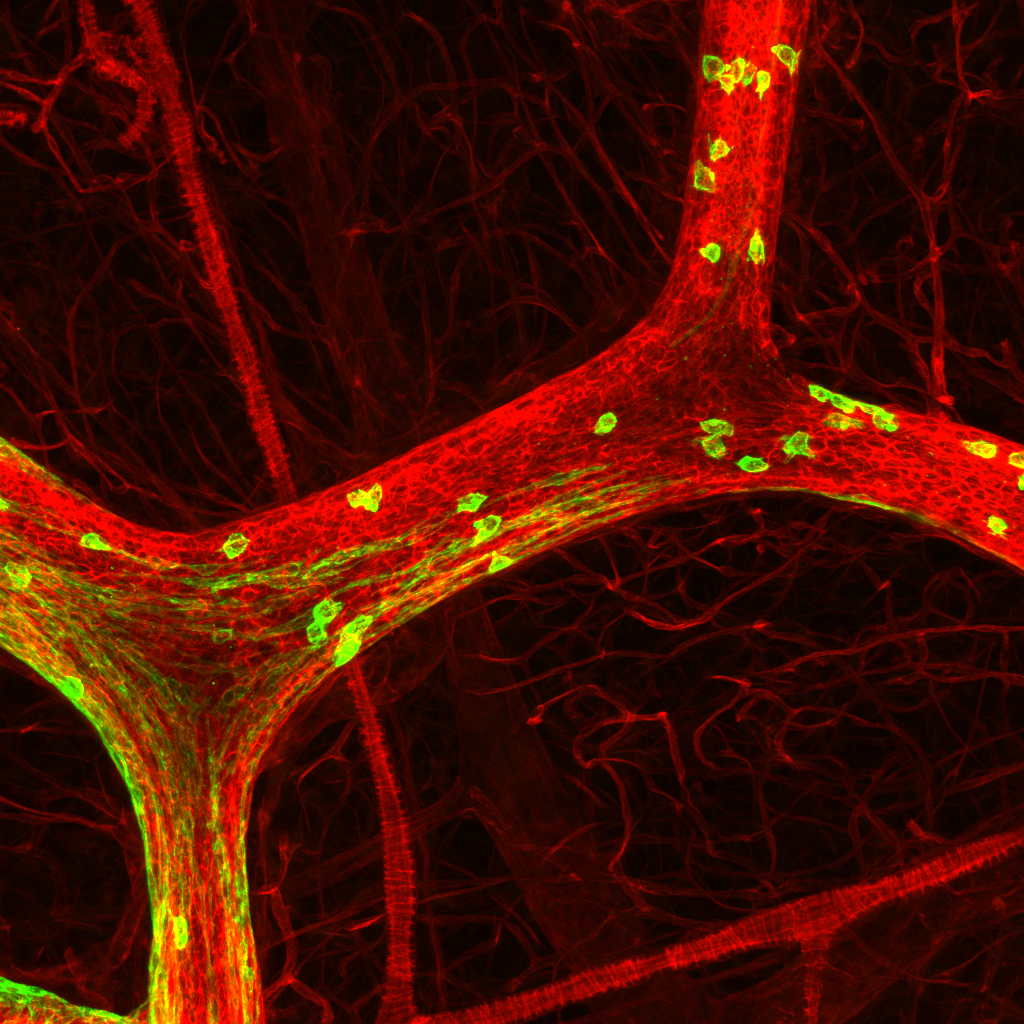
Disease models:
Triple Negative Breast Cancer (TNBC): TNBC is the most aggressive subtype of breast cancer, lacking well defined driver lesions that could inform targeted therapies. Leveraging our in-utero lentivirus injection based genetic screen system, we functionally identified major driver combinations from hundreds of gain- and loss-of-function lesions enriched in TNBC patients. Our ongoing research investigates how these driver lesions hijack normal mammary developmental programs to fuel TNBC specific cell fate commitment.
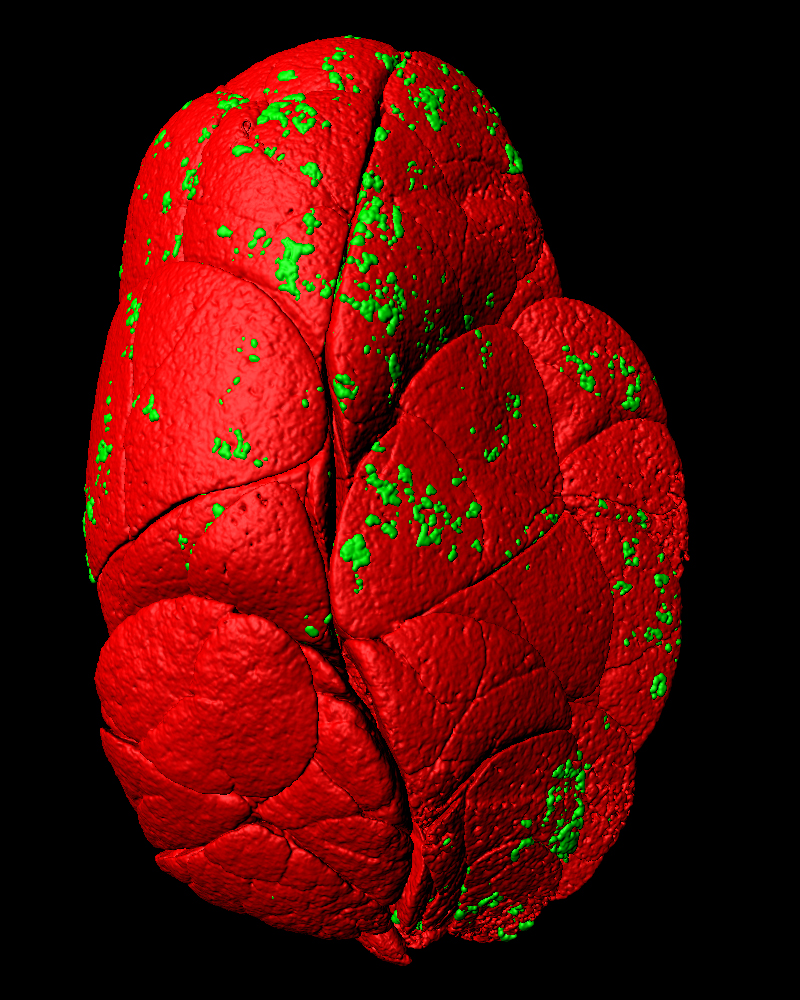
Targeted regeneration post radiation therapy: cancer therapies like irradiation inevitably damage normal tissue, while therapeutic activation of major protective and regenerative pathways often increases the risk of cancer recurrence. Using oral cancer and the adjacent radiosensitive salivary glands as models, we have identified molecular approaches to selectively replenish damaged salivary glands without stimulating cancer recurrence. Our ongoing work aims to establish a framework for understanding the distinct growth control mechanisms in normal versus cancerous epithelium post irradiation.
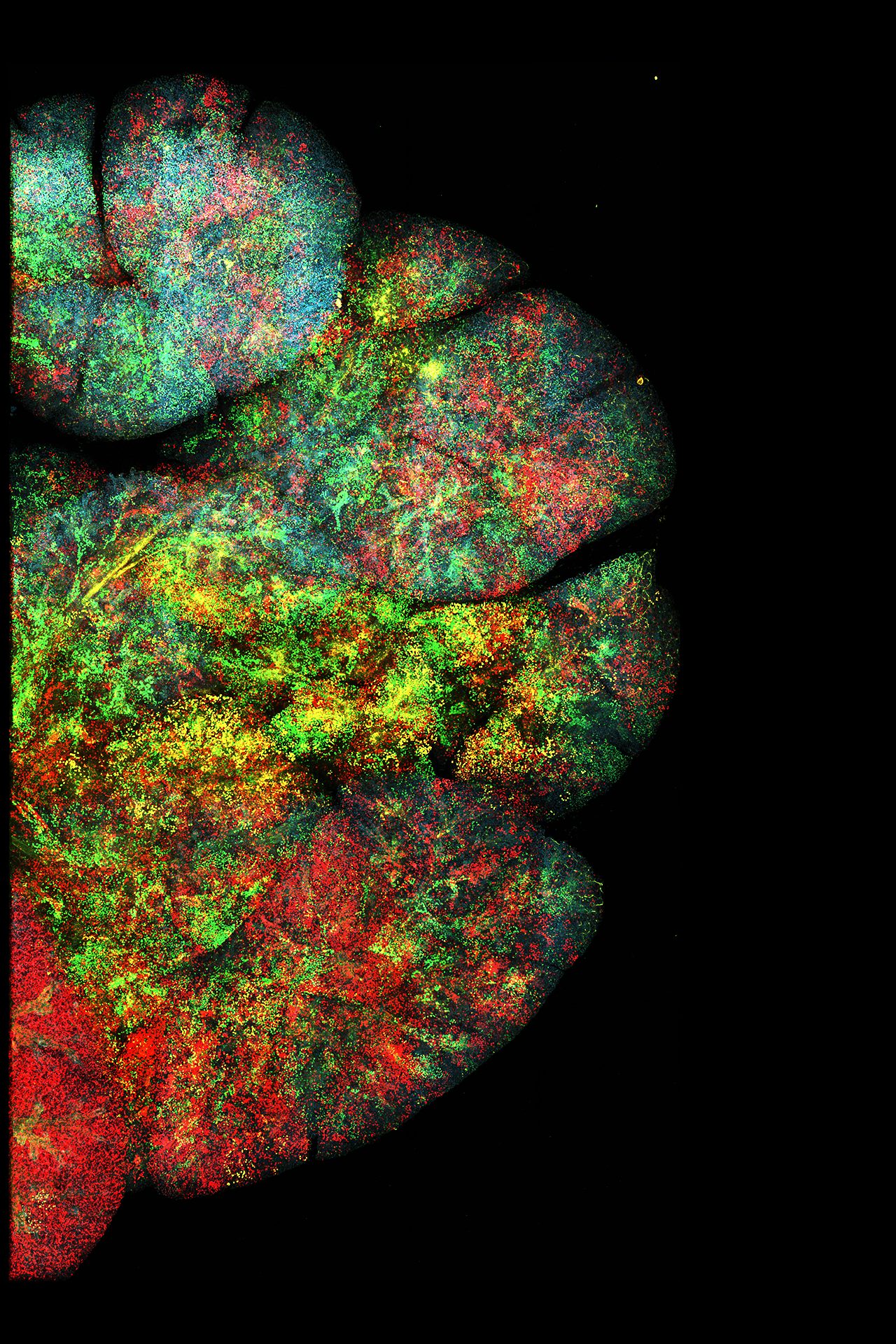
Mission and approach:
Efficient, Precise Epithelial Disease Modeling: To address critical challenges in our disease models, we continue to advance gene targeting systems that enable rapid, large scale functional testing in glandular and stratified epithelia. Our in-utero lentivirus injection based approaches allow us to go beyond the traditional “one strain, one condition” genetic models, allowing hundreds of genetic conditions to be tested within intact organs, such as the mammary and salivary glands, in a single mouse. This high-throughput approach allows us to prioritize key mechanisms with unprecedented efficiency. Coupling advanced imaging techniques with high-fidelity barcoded lineage tracing, we investigate cell fate decisions at tissue, clonal, and single-cell resolutions. In collaboration with clinicians and industry, we validate our discoveries in patient-derived models and explore potential therapeutic applications.

Training the Next Generation of Innovators: We aim to equip trainees with the skills and mindset necessary to excel in both academia and industry. We emphasize critical thinking, innovative problem solving, and a focus on impactful discovery. In addition to developing research expertise, trainees receive dedicated guidance from the PI in scientific writing, grantsmanship and presentation skills. Through partnerships with clinical and industry collaborators, they also gain real-world translational experience, preparing them for diverse career paths that maximize their intellectual potential.
Our Sponsors

