RESEARCH
We study how cells feel and respond to force—then we use that to rebuild functional muscle.
Bioengineering Strategies for Treating Rotator Cuff Muscle Disease
Rotator cuff tendon injuries are common and affect over 30% of the adult population. While surgical reattachment procedures are routinely performed, failure and re-tear rates remain high and worsen with aging. This is attributed to progressive degeneration of the associated muscles characterized by atrophy and intramuscular fatty infiltration, where fatty infiltration is caused by aberrant adipogenesis of the muscle-resident fibro-adipogenic progenitors. We recently determined that Wnt7a effectively suppresses the adipogenic potential of fibro-adipogenic progenitors through YAP/TAZ signaling. To this end, we are currently working to (1) unveil the roles of Wnt and mechanotransduction pathways in regulating fibro-adipogenic progenitor fates, and (2) develop hydrogel-based strategies for combating intramuscular fatty infiltration and atrophy in rotator cuff muscle disease in the context of aging.
Santiago, LM; Oguntuyo, K; Chin-Young, B; Fang, F; Amabile, A; Han, WM “WNT7A mRNA Lipid Nanoparticles Promote Muscle Hypertrophy and Reduce Skeletal Muscle Fatty Infiltration,” Cellular and Molecular Bioengineering, 2025, In Press.
Fu, C; Chin-Young, B; Park, G; Guzman-Seda, M; Laudier, D; Han, WM “WNT7a Suppresses Adipogenesis of Skeletal Muscle Mesenchymal Stem Cells and Fatty Infiltration Through the Alternative Wnt-Rho-YAP/TAZ Signaling Axis” Stem Cell Reports, 2023, 18(4): P999-1014.
Fu, C; Huang, AH; Galatz, LM; Han, WM “Cellular and Molecular Modulation of Rotator Cuff Muscle Pathophysiology” Journal of Orthopaedic Research, 2021, 39:2310-2322.
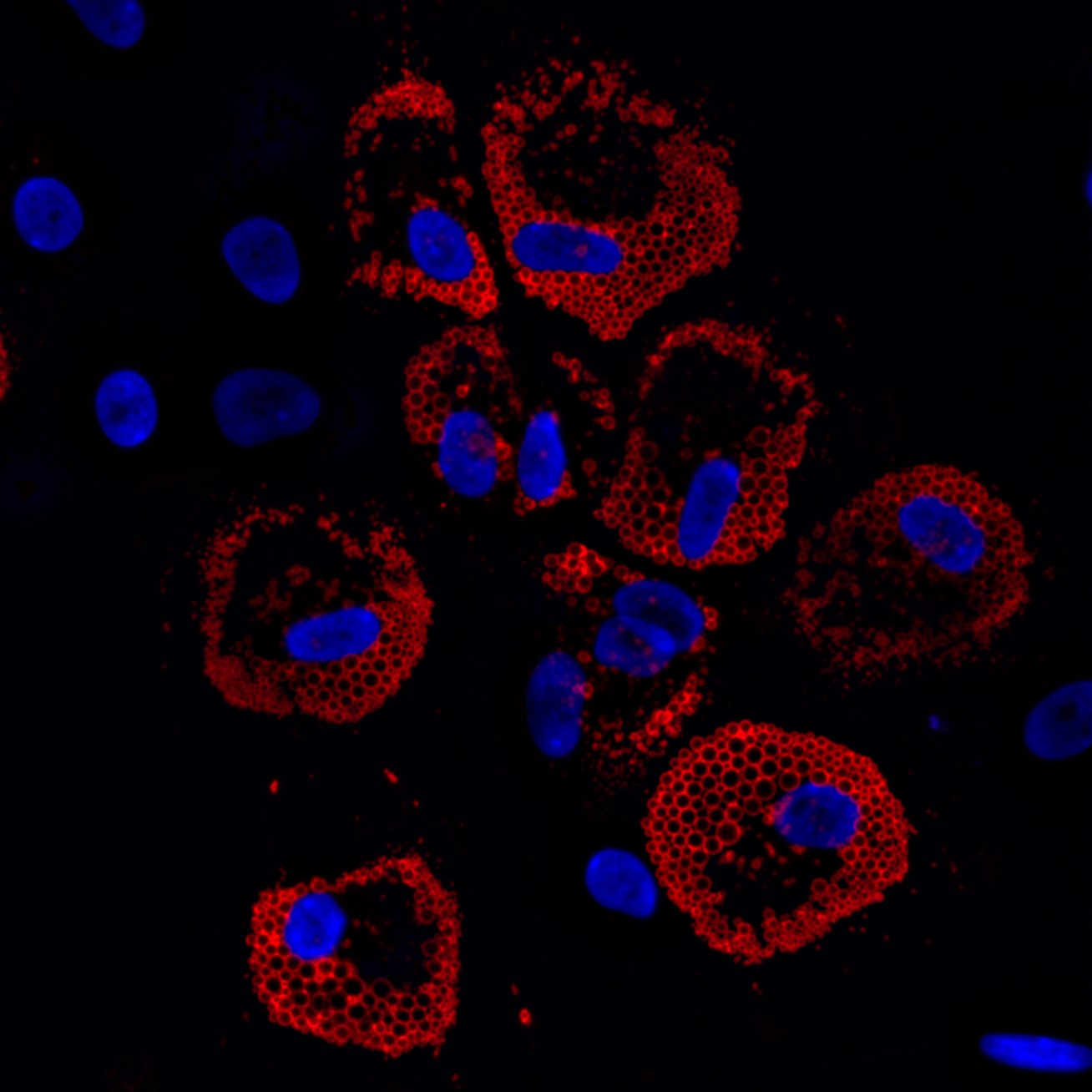
Cell-Instructive Biomaterials for Muscle Stem/Satellite Cell Manufacturing & Transplantation
Muscle stem cell transplantation is a promising strategy to treat skeletal muscle injuries and diseases, but a direct injection of cells results in poor donor cell survival, engraftment, and function of transplanted cells. Methods to maintain and expand therapeutically potent muscle stem cells ex vivo for clinical translation also remain a significant challenge. To this end, we are engineering cell-instructive biomaterial-based technologies to (1) facilitate the transplantation of muscle stem cells, (2) augment muscle regeneration in musculoskeletal injuries and diseases, and (3) systematically understand how muscle stem cells integrate biophysical & biochemical cues derived from their geometrically asymmetric niche to regulate their function.
Han, WM; Mohiuddin, M; Anderson, SE; García, AJ*; Jang, YC* “Co-delivery of Wnt7a and Muscle Stem Cells using Synthetic Bioadhesive Hydrogel Enhances Murine Muscle Regeneration and Cell Migration during Engraftment” Acta Biomaterialia, 2019, 96, 243-252. *Co-PI. PMID: 31228633.
Han, WM; Anderson, SE; Mohiuddin, M; Barros, D; Nakhai, SA; Shin, E; Amaral, IF; Pêgo, AP; García, AJ*; Jang, YC* “Synthetic Matrix Enhances Transplanted Satellite Cell Engraftment in Dystrophic and Aged Skeletal Muscle with Comorbid Trauma” Science Advances, 2018, 4:eaar4008. *Co-PI. PMID: 30116776.
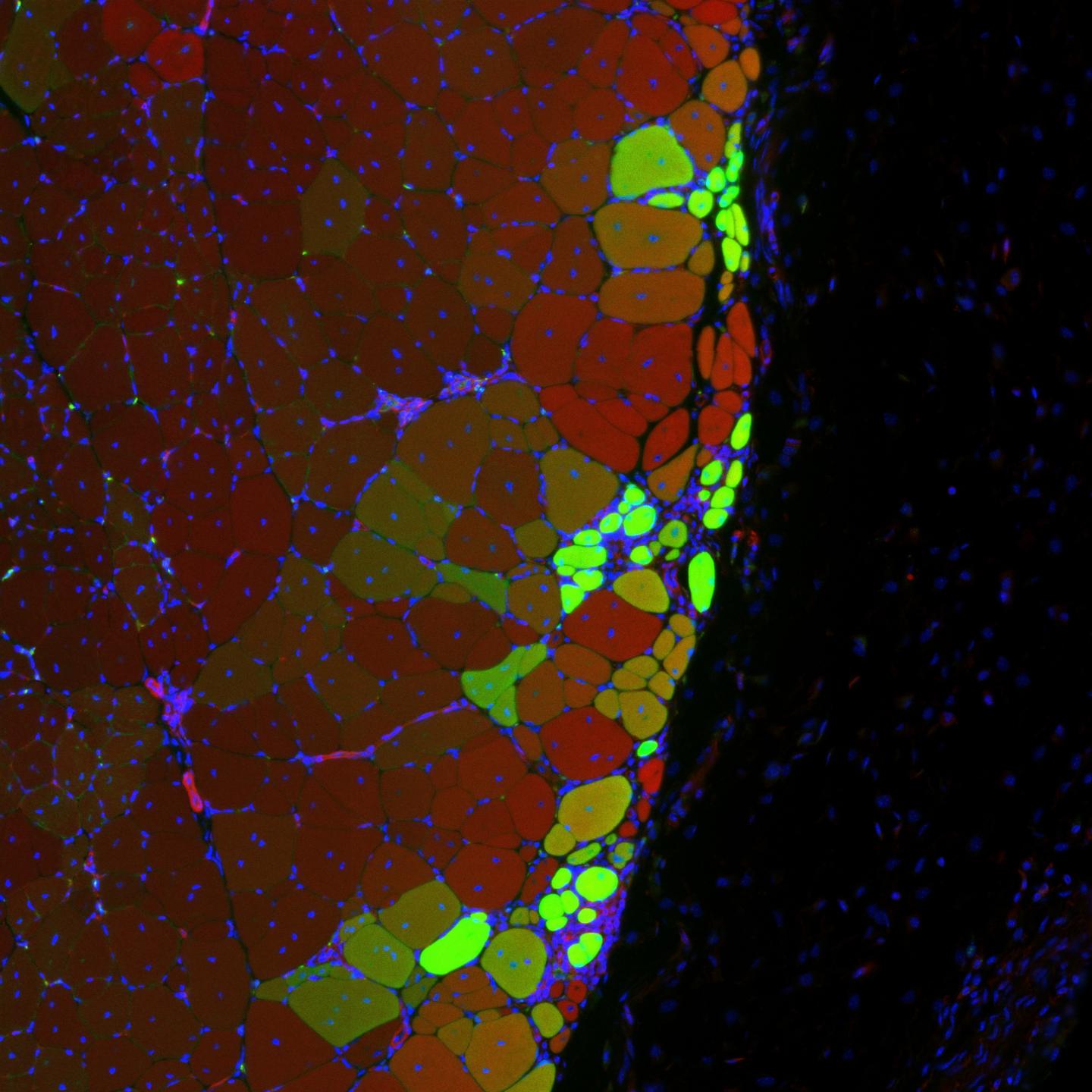
Musculoskeletal Mechanobiology
Physical forces play a critical role in regulating cell function, particularly in mechanically dynamic musculoskeletal tissues. To understand how cells sense and respond to these forces across multiple length scales, we integrate in vivo models, native tissues, and engineered biomaterials. Our work has explored how externally applied strain is transmitted from the tissue level to resident cells, influencing early mechanotransductive responses in fiber-reinforced soft tissues. More recently, we have investigated how 3D mechanical confinement regulates muscle stem cell (MuSC) fate and function, revealing its role as a mechanical brake on MuSC activation. By leveraging biomaterial platforms that mimic key biophysical features of native tissues, we can systematically dissect the interplay between mechanical forces, microenvironmental constraints, and cellular behavior. This approach enables us to study mechanobiological regulation in both healthy and pathological states, advancing our understanding of how physical cues shape cell function and tissue regeneration.
Park, G; Grey, JA; Mourkioti, F; Han, WM “3D Mechanical Confinement Directs Muscle Stem Cell Fate and Function” Advanced Biology, 2025, 9 (4), 2570098. PMID: 40040295.
Han, WM∇; Heo, SJ∇; Driscoll, DP; Delucca, JF; McLeod, CM; Smith, LJ; Duncan, RL; Mauck, RL*; Elliott, DM* “Microstructural heterogeneity directs micromechanics and mechanobiology in native and engineered fibrocartilage” Nature Materials, 2016, 15, 477-484. ∇Co-Authors. *Co-PI. PMID: 26726994.
Han, WM; Heo, SJ; Driscoll, TP; Mauck, RL; Smith, LJ; Elliott, DM “Macro to Micro-scale Strain Transfer in Fibrous Tissues is Heterogeneous and Tissue-Specific” Biophysical Journal, 2013, 105, 807-817. PMID: 23931328.

Our Sponsors
Current Funding



Past Funding



Han Laboratory
Woojin Han, PhD
Associate Professor
Department of Orthopaedics
Department of Stem Cell Biology & Regenerative Medicine
Location
Lab: Annenberg A20-40
Office: Annenberg A20-66A
Contact
Phone: 212-241-4507
Email: woojin.han -at- mssm.edu
Bluesky: @thewooj.bsky.social
Follow us
