SARS-CoV-2
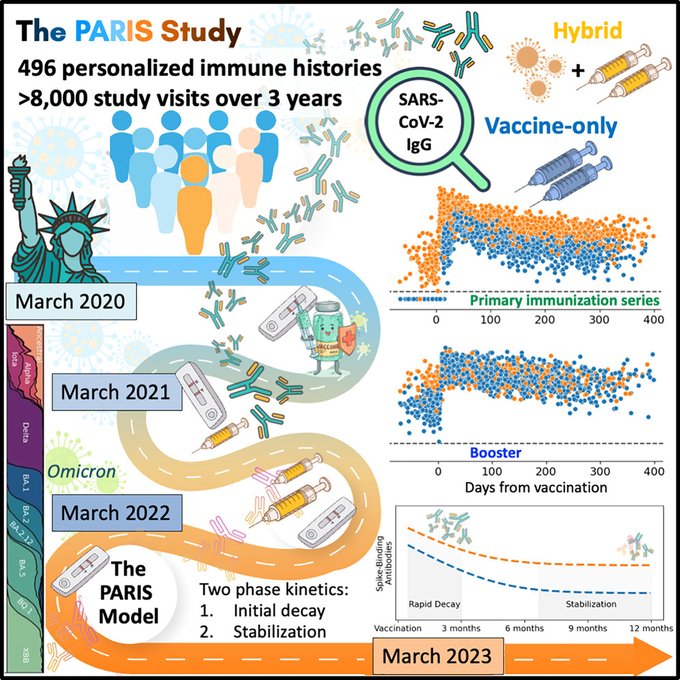
SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase.
Immunity. 2024 Mar 12;57(3):587-599.e4. doi: 10.1016/j.immuni.2024.01.017. Epub 2024 Feb 22.PMID: 38395697 Free PMC article.
Graphical Abstract:
A surrogate ELISA to select high titer human convalescent plasma for treating immunocompromised patients infected with SARS-CoV-2 variants of concern.
J Infect Dis. 2025 Jan 3:jiae645. doi: 10.1093/infdis/jiae645. Online ahead of print.PMID: 39749487
Nirmatrelvir and molnupiravir maintain potent in vitro and in vivo antiviral activity against circulating SARS-CoV-2 omicron subvariants.
Antiviral Res. 2024 Oct;230:105970. doi: 10.1016/j.antiviral.2024.105970. Epub 2024 Jul 25.PMID: 39067667
SARS-CoV-2 serosurvey across multiple waves of the COVID-19 pandemic in New York City between 2020-2023.
Nat Commun. 2024 Jul 11;15(1):5847. doi: 10.1038/s41467-024-50052-2.PMID: 38992013 Free PMC article.
Influenza
The immunodominance of antigenic site Sb on the H1 influenza virus hemagglutinin increases with high immunoglobulin titers of the cohorts and with young age, but not sex.
Vaccine. 2024 May 22;42(14):3365-3373. doi: 10.1016/j.vaccine.2024.04.037. Epub 2024 Apr 15.PMID: 38627145 Free article.
Bat-borne H9N2 influenza virus evades MxA restriction and exhibits efficient replication and transmission in ferrets.
Nat Commun. 2024 Apr 25;15(1):3450. doi: 10.1038/s41467-024-47455-6.PMID: 38664395 Free PMC article.
Mpox
Development of a novel serological assay for the detection of mpox infection in vaccinated populations.
J Med Virol. 2023 Oct;95(10):e29134. doi: 10.1002/jmv.29134.PMID: 37805977 Free PMC article.
Mpox vaccine and infection-driven human immune signatures: an immunological analysis of an observational study.
Lancet Infect Dis. 2023 Nov;23(11):1302-1312. doi: 10.1016/S1473-3099(23)00352-3. Epub 2023 Jul 17.PMID: 37475115 Free PMC article.
Genomic and ultrastructural analysis of monkeypox virus in skin lesions and in human/animal infected cells reveals further morphofunctional insights into viral pathogenicity.
J Med Virol. 2023 Jun;95(6):e28878. doi: 10.1002/jmv.28878.PMID: 37322614
HIV
Development of an HIV reporter virus that identifies latently infected CD4+ T cells.
Cell Rep Methods. 2022 Jun 13;2(6):100238. doi: 10.1016/j.crmeth.2022.100238. eCollection 2022 Jun 20.PMID: 35784650 Free PMC article.
Global post-translational modification profiling of HIV-1-infected cells reveals mechanisms of host cellular pathway remodeling.
Cell Rep. 2022 Apr 12;39(2):110690. doi: 10.1016/j.celrep.2022.110690.PMID: 35417684 Free PMC article.
HERVs
An integrated approach for the accurate detection of HERV-K HML-2 transcription and protein synthesis.
Nucleic Acids Res. 2025 Jan 11;53(2):gkaf011. doi: 10.1093/nar/gkaf011.PMID: 39831303 Free PMC article.