PUBLICATIONS
Selected Publications:
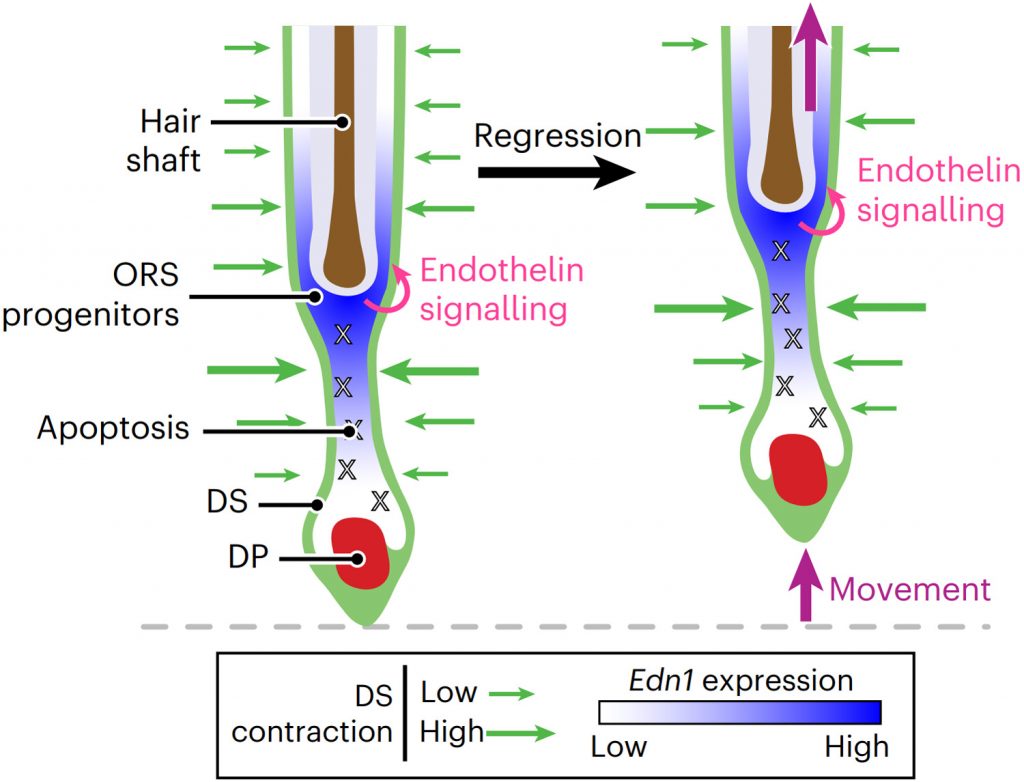
 Progenitor-derived endothelin controls dermal sheath contraction for hair follicle regression
Progenitor-derived endothelin controls dermal sheath contraction for hair follicle regression
Pieter Martino*, Raghava Sunkara*, Nicholas Heitman, Martina Rangl, Alexia Brown, Nivedita Saxena, Laura Grisanti, Donald Kohan, Masashi Yanagisawa, Michael Rendl
Nature Cell Biology (2023) pdf
Substantial follicle remodelling during the regression phase of the hair growth cycle is coordinated by the contraction of the dermal sheath smooth muscle, but how dermal-sheath-generated forces are regulated is unclear. Here, we identify spatiotemporally controlled endothelin signalling—a potent vasoconstriction-regulating pathway—as the key activating mechanism of dermal sheath contraction. Pharmacological blocking or genetic ablation of both endothelin receptors, ETA and ETB, impedes dermal sheath contraction and halts follicle regression. Epithelial progenitors at the club hair–epithelial strand bottleneck produce the endothelin ligand ET-1, which is required for follicle regression. ET signalling in dermal sheath cells and downstream contraction is dynamically regulated by cytoplasmic Ca2+ levels through cell membrane and sarcoplasmic reticulum calcium channels. Together, these findings illuminate an epithelial–mesenchymal interaction paradigm in which progenitors—destined to undergo programmed cell death—control the contraction of the surrounding sheath smooth muscle to orchestrate homeostatic tissue regression and reorganization for the next stem cell activation and regeneration cycle.
Research Briefing
Nature Cell Biology 25, 220–221 (2023) pdf
In The News
Improved understanding of pathways could lead to new hair loss therapies
Mount Sinai Researchers Identify Details of Muscle’s Role in Regulating Hair Growth Cycle
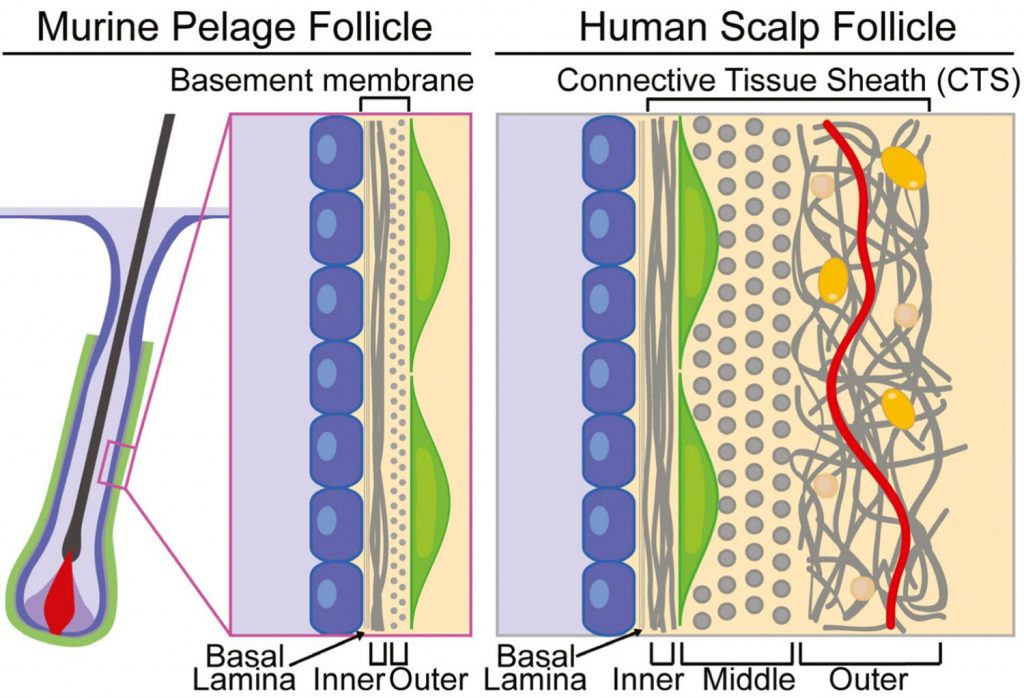
 The dermal sheath: An emerging component of the hair follicle stem cell niche
The dermal sheath: An emerging component of the hair follicle stem cell niche
Pieter Martino, Nicholas Heitman, Michael Rendl
Experimental Dermatology (2021) pdf
Hair follicles cyclically regenerate throughout adult mammalian life, owing to a resident population of epithelial hair follicle stem cells. Stem cell (SC) activity drives bouts of follicle growth, which are periodically interrupted by follicle regression and rest. These phases and the transitions between them are tightly spatiotemporally coordinated by signalling crosstalk between stem/progenitor cells and the various cell types of the microenvironment, or niche. The dermal papilla (DP) is a cluster of specialized mesenchymal cells that have long been recognized for important niche roles in regulating hair follicle SC activation, as well as progenitor proliferation and differentiation during follicle growth. In addition to the DP, the mesenchyme of the murine pelage follicle is also comprised of a follicle-lining smooth muscle known as the dermal sheath (DS), which has been far less studied than the DP yet may be equally specialized and important for hair cycling. In this review, we define the murine pelage DS in comparison with human DS and discuss recent work that highlights the emergent importance of the DS in the hair follicle SC niche. Last, we examine potential therapeutic applications for the DS in hair regeneration and wound healing.
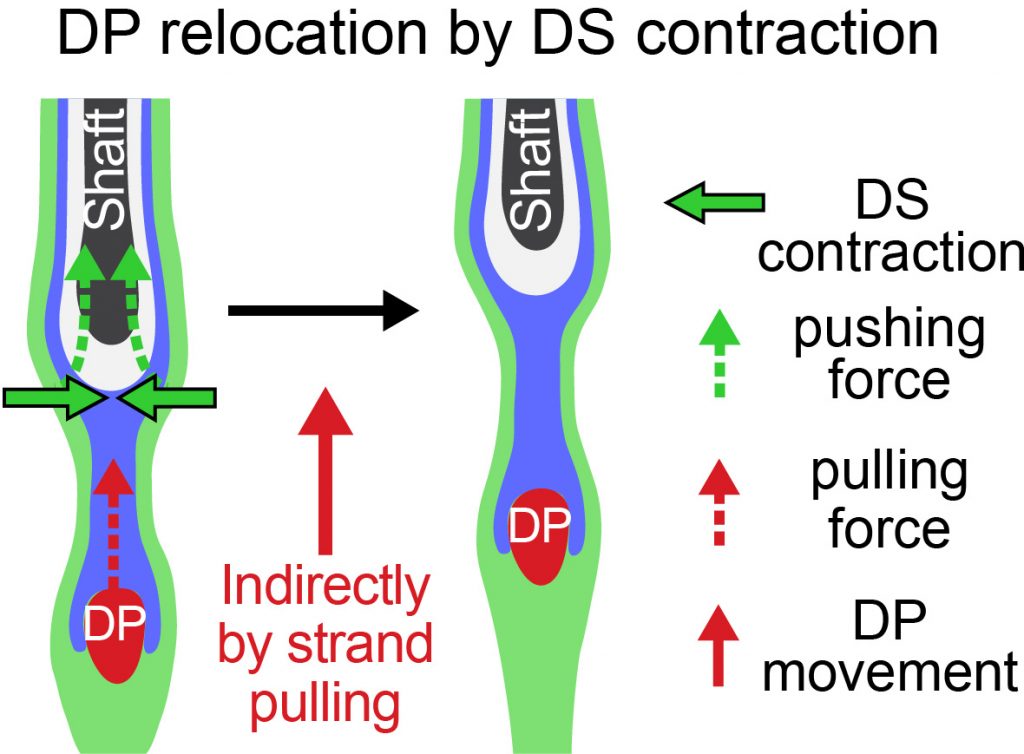
 Dermal sheath contraction powers stem cell niche relocation during hair cycle regression
Dermal sheath contraction powers stem cell niche relocation during hair cycle regression
Nicholas Heitman, Rachel Sennett, Ka-Wai Mok, Nivedita Saxena, Devika Srivastava, Pieter Martino, Laura Grisanti, Zichen Wang, Avi Ma’ayan, Pantelis Rompolas, Michael Rendl
Science (2020) pdf
Tissue homeostasis requires the balance of growth by cell production and regression through cell loss. In the hair cycle, during follicle regression, the niche traverses the skin through an unknown mechanism to reach the stem cell reservoir and trigger new growth. Here, we identify the dermal sheath that lines the follicle as the key driver of tissue regression and niche relocation through the smooth muscle contractile machinery that generates centripetal constriction force. We reveal that the calcium-calmodulin–myosin light chain kinase pathway controls sheath contraction. When this pathway is blocked, sheath contraction is inhibited, impeding follicle regression and niche relocation. Thus, our study identifies the dermal sheath as smooth muscle that drives follicle regression for reuniting niche and stem cells in order to regenerate tissue structure during homeostasis.