RESEARCH
TISSUE REGRESSION AND REORGANIZATION
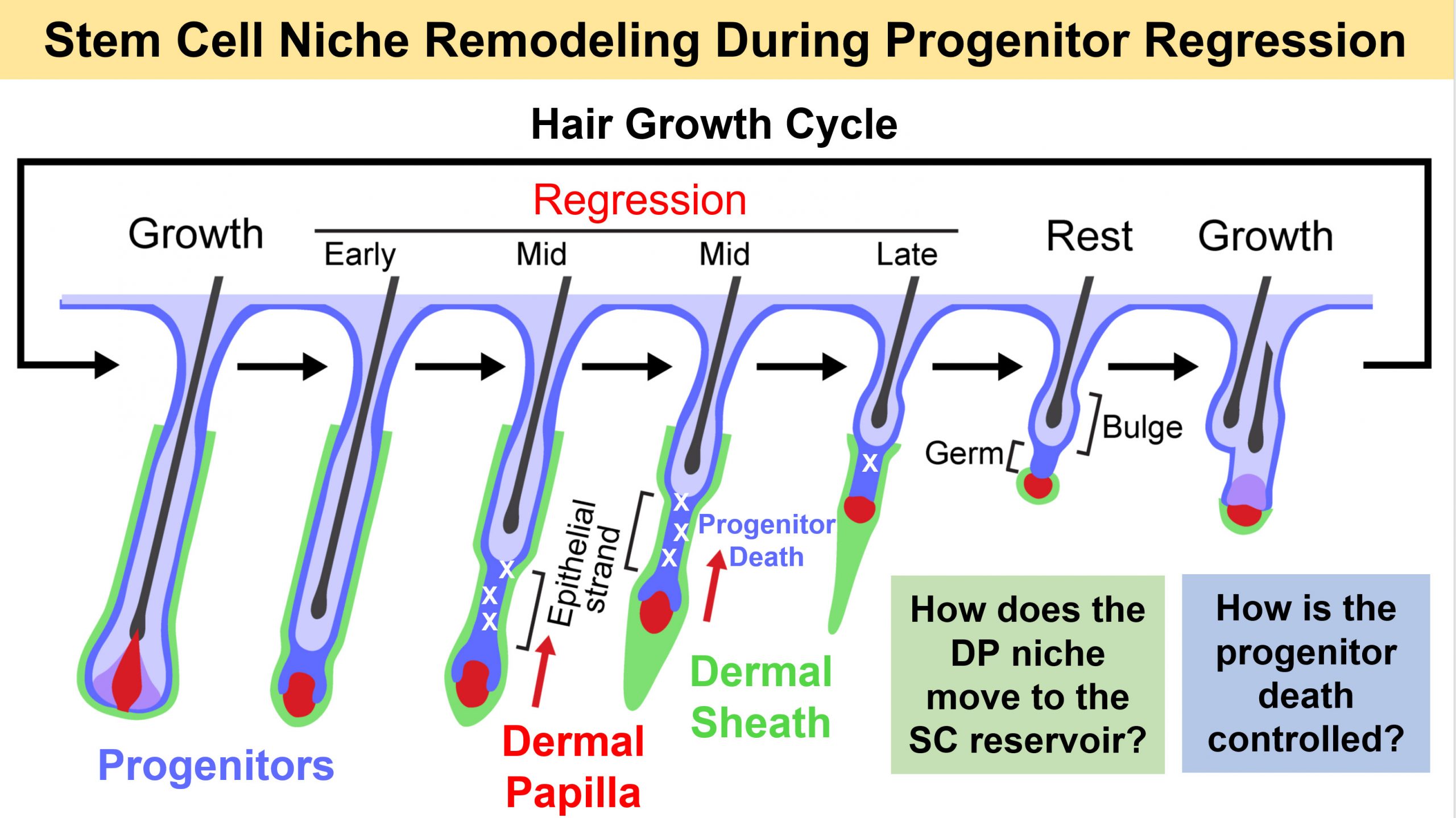
How tissues regenerate from stem cells and how different organs balance cell loss with new growth is under intensive investigation in the regenerative biology field. In the natural hair growth cycle after years of continuous hair growth, follicles enter a wave of massive progenitor death along the hair axis that – as we are just beginning to unravel – is controlled by the niche microenvironment.
During follicle regression, the Dermal Papilla niche relocates from the base of growing hair bulbs to the stem cell reservoir in the upper follicle for regeneration and new hair growth. We have recently uncovered that the Dermal Sheath – follicle-lining cells with previously unidentified physiological functions (Martino et al, Experimental Dermatology 2021) – is a smooth muscle that contracts to extrude the hair shaft and relocate the niche to its stem cell-adjacent position (Heitman et al, Science 2020). Progenitors at the follicle bottleneck region regulate sheath contraction via endothelin signaling (Martino et al, Nature Cell Biology 2023). New insights lead us now to hypothesize that the Dermal Sheath is a previously unknown signaling source that directly regulates the controlled death of progenitor. Many important questions have arisen:
1) What are the molecular controls in Sheath cells and Progenitors that initiate and regulate contraction?
2) Is the Dermal Sheath a signaling niche in its own right that controls progenitor cell fate?
3) What are the molecular mechanisms that control Dermal Sheath identity and turnover?
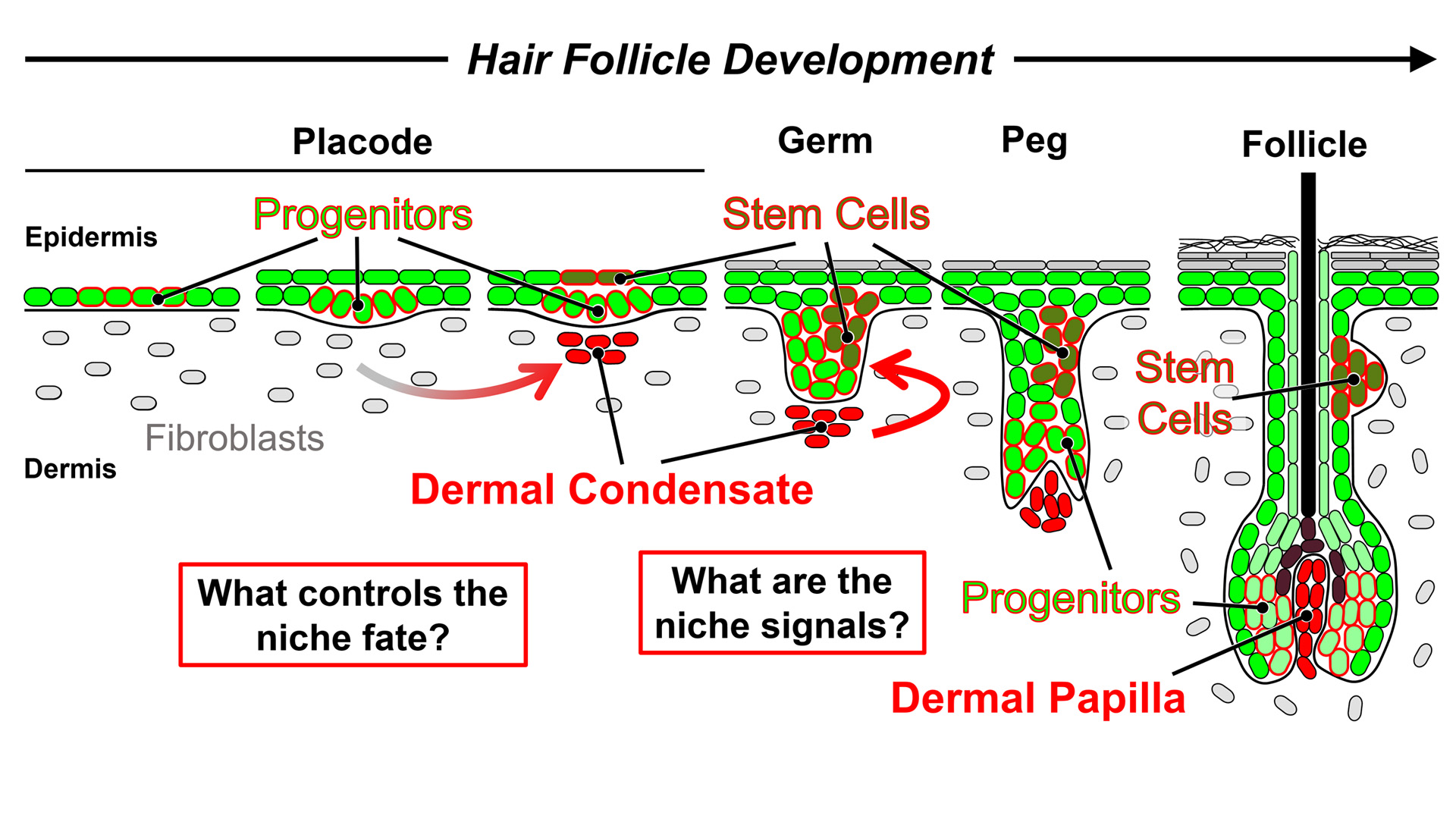
BIRTH OF THE HAIR FOLLICLE NICHE
A central question in stem cell research is how during organ development initially naïve and unspecialized cells turn into the stem cells and instructive niche cells that regenerate tissue in the adult. During embryonic hair follicle formation, the progenitors in nascent hair placodes of the epidermal layer receive niche signals from cell clusters underneath that are called Dermal Condensates. Dermal Condensate cells are the original stem cell-activating niche in the developing skin that controls the downgrowth of follicle stem cells and progenitors. A major mission of the lab is to unlock the secrets of how the condensate niche regulates these processes.
Towards this goal, we utilize transcriptomics, organoid technology, live imaging of skin and forward genetics for gene function studies. We generated the foundational gene expression knowledge of the dermal condensate niche and progenitors (Sennett et al, Developmental Cell 2015) – shared in a popular online database (HAIR-GEL.net) – and established gene targeting drivers (Grisanti et al, Journal of Investigative Dermatology 2013) for functional insights (Clavel et al, Developmental Cell 2012, Tsai et al, Developmental Biology 2014). We are particularly interested in how the molecular transitions are regulated from naïve fibroblasts into specialized condensates (Mok et al, Developmental Cell 2019) that hold the key to niche fate specification (Saxena et al, Experimental Dermatology 2019). Major questions are:
1) What are the transcriptional controls of niche cell fate specification?
2) What are the inductive niche signals?
3) What are the cell sorting mechanisms during niche formation?
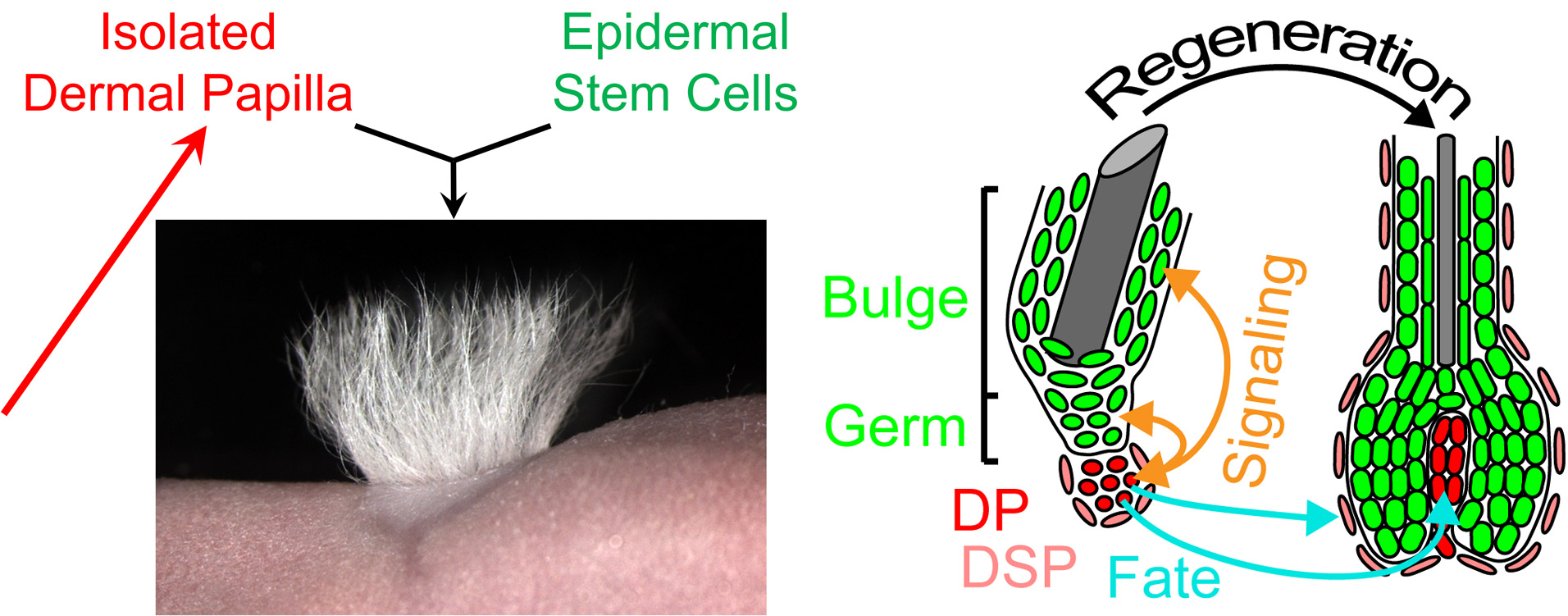
GROWTH CONTROL AND REGENERATION
How is tissue growth, homeostasis and renewal by stem cells regulated throughout life? Understanding the core molecular controls is fundamental for realizing the therapeutic potential of the instructive niche (Rezza et al, Current Topics in Developmental Biology 2014). After morphogenesis, the Dermal Condensate gives rise to the inductive niche of adult hair follicles, the Dermal Papilla. Dermal Papilla cells signal to progenitors in the follicle bulb to produce and grow hair shafts. The Dermal Papilla is also critical for activating stem cells to regenerate a new follicle during each hair growth cycle. Isolated papilla niche cells can induce new hair formation after cell transplantation into skin in mice, a yet unrealized goal in human.
To uncover the niche controls we previously defined the signature gene expression of dermal papillae from different growing hair follicle types (Rezza et al, Cell Reports 2016) and – with still unpublished work – of papillae and stem cells from follicles during the rest-to-regeneration transition. With novel niche-specific genetic drivers we are now in the prime position to uncover the functions of uniquely expressed niche genes. Key questions are:
1) How does the Dermal Papilla regulate progenitor proliferation, migration and differentiation?
2) What are the molecular controls of the Dermal Papilla inductive fate?
3) What are the niche inductive signals to the stem cells?