The mechanism of ubiquitination by the Cullin-RING E3 ubiquitin ligases
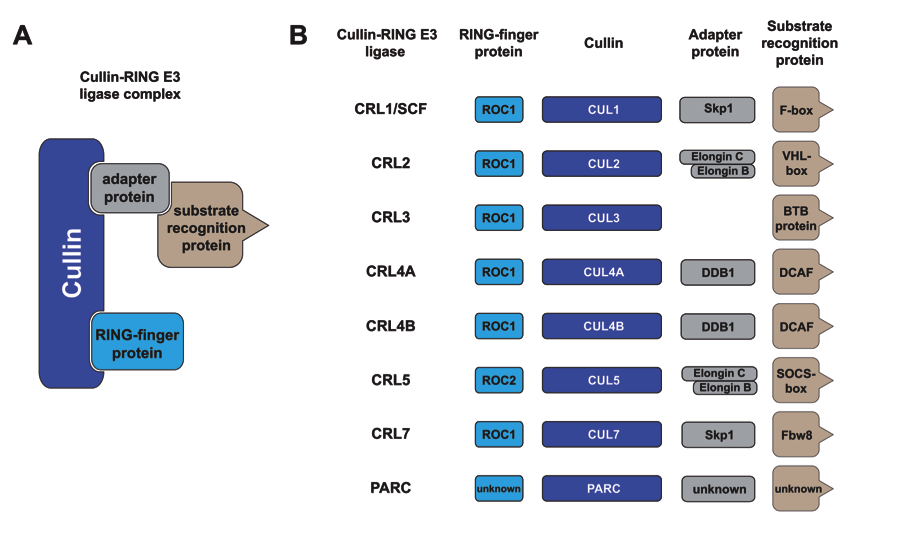
Cullin-RING E3 ubiquitin ligases (CRLs) are the largest RING-type of E3 family consisting of ≈240 members, nearly half of E3s identified in humans. CRL is responsible for ~20% of protein degradation mediated by the 26S proteasome. CRL dys-regulation contributes directly to human diseases including cancer. Canonical CRLs are modular complexes, in which a cullin’s N terminal domain assembles interchangeably with different CUL-specific substrate receptors capable of binding a substrate. On the other hand, the C-terminal half of a cullin (cullin CTD) binds a RING finger protein, ROC1/RBX1 for CUL1-CUL4 or ROC2/RBX2 for CUL5, to form a core ligase complex. CRL’s core ligase can collaborate with specific E2 conjugating enzymes to catalyze the transfer of Ub to the bound substrate and the subsequent polyubiquitination reaction. A major focus of our lab is to explore mechanistic insights into the polyubiquitination reaction orchestrated by CRL.
