Research and Projects
Understanding the molecular and cellular mechanisms regulating the development, patterning and postnatal renewal of the skin and ectodermal appendage organs such as hair follicles, teeth, and taste papillae, and identifying stem and progenitor cell populations in these organs, is critical for developing new therapies to accelerate wound healing, treat hair loss diseases, repair or replace diseased teeth, and ameliorate taste dysfunction.
Research in the Millar lab focuses on cell-cell signaling and epigenetic mechanisms that underlie these processes. In published research, we identified Wnt/beta-catenin signaling as a key pathway required for initiating the formation of ectodermal appendages from multipotent cells in mammalian embryos, and in controlling development and patterning of haired versus hairy skin. By analyzing genetic mouse models and tissues from human patients carrying mutations in the WNT10A gene, we showed that Wnt signaling plays key roles in regulating the functions of a wide variety of adult epithelial stem cells, as well as controlling specialized differentiation programs in palmoplantar skin. We have also identified critical functions for epigenetic regulators including micro-RNAs and chromatin modifiers in skin development and regeneration.
Our current research projects include:
1: Investigating mechanisms that cause ectodermal dysplasia in patients with mutations in the WNT10A gene and testing potential therapeutic strategies.
We identified WNT10A as a key ligand controlling epithelial stem cell proliferation and region-specific differentiation, providing a molecular basis for understanding ectodermal dysplasia and regenerative defects in WNT10A mutant patients. We are further exploring the mechanisms underlying palmoplantar keratoderma, the most clinically relevant skin defect in WNT10A patients, and testing potential therapeutic approaches using pre-clinical models and patient-derived and gene-edited induced pluripotent stem cells (iPS).
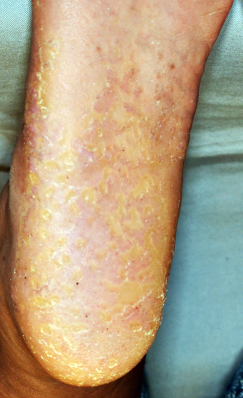
Palmoplantar keratoderma in a patient homozygous for the WNT10A c.756+1G>A mutation.
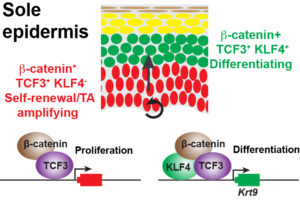
Model for the mechanisms downstream of WNT10A that regulate proliferation and differentiation in sole epidermis.
2: Determining the mechanisms that underlie the formation and maintenance of hairy versus hairless skin and regulate hair patterning.
Different regions of mammalian skin vary in their functions, regenerative properties, and responses to injury and disease. Delineating the mechanisms that establish and maintain skin heterogeneity has potential to reveal improved therapeutic strategies. Positional information resides in the skin dermis, but the responsible molecular signals are poorly defined. We are using regional variation of hair follicle development and regeneration as a model system to address this question. We are particularly focusing on endogenous Wnt inhibitors as mediators of regional variation and hair patterning in the skin; our initial studies have identified the Dickkopf2 (DKK2) inhibitor as playing a critical role in permitting development of hairless plantar skin.
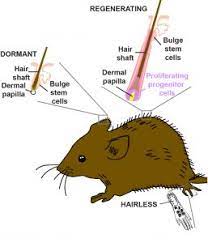
Different regions of mouse skin contain regenerative hair follicles, dormant hair follicles, or no hair follicles.
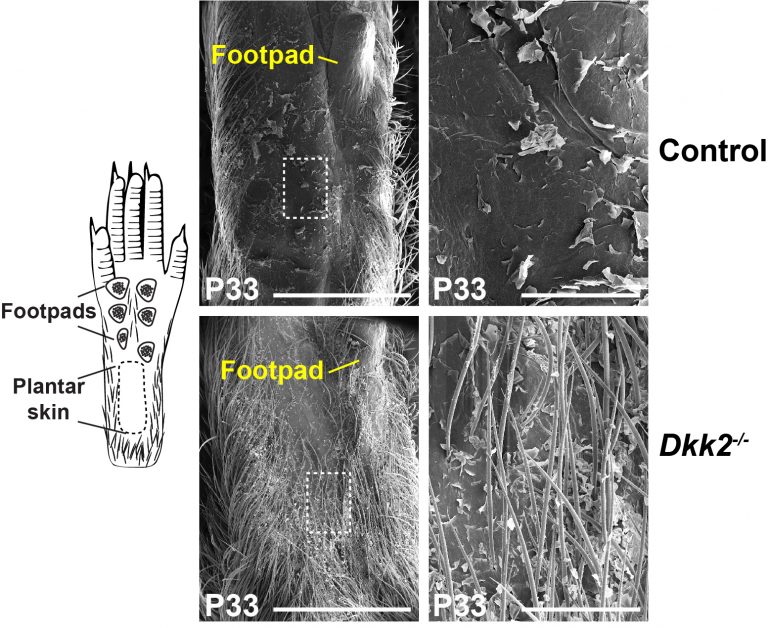
Plantar skin in mice is normally hairless, but grows hair in mice with a loss of function mutation in the Dickkopf2 (Dkk2) gene, which encodes an endogenous Wnt inhibitor.
3: Defining the functions of histone deacetylase chromatin modifiers in skin development, stem cells and cancer.
Embryonic development and postnatal renewal of the epidermis and hair follicles require coordinated changes in gene expression that are often dysregulated in tumorigenesis. Histone deacetylases (HDACs) provide global control of gene expression programs by repressive modification of chromatin, and form several classes, of which Class 1 members display the highest histone deacetylase activity. Of these, HDACs 1 and 2 associate with Mi2b, MTA-2 and MDB3 in the NuRD complex and with Sin3, SAP18 and SAP30 in the Sin3 complex, while HDAC3 complexes with N-CoR or SMRT. HDAC inhibitors are promising therapeutic agents for multiple types of tumor. However, while the functions and targets of individual HDACs vary widely, most HDAC inhibitors broadly block Class 1 HDACs, and their precise mechanisms of action are poorly understood. We are using genetic, biochemical, and global genomic approaches to delineate and compare the functions of HDAC1, HDAC2 and HDAC3 in the development and regeneration of skin epithelia, to identify transcription factors that target individual HDACs to specific sets of promoters in skin epithelial cells, and to determine the consequences of deletion of Hdac1, 2 or 3 for the initiation and progression of skin tumors. Our published studies showed that HDAC1 and HDAC2 function redundantly to permit self-renewal and survival of embryonic epidermal stem cells. By contrast, we find that HDAC3 is necessary for orderly stepwise differentiation of the epidermis. Data from these experiments will be critical for evaluating the potential risks and benefits of targeting individual HDACs in the epidermis and for developing more specific and effective therapeutics.
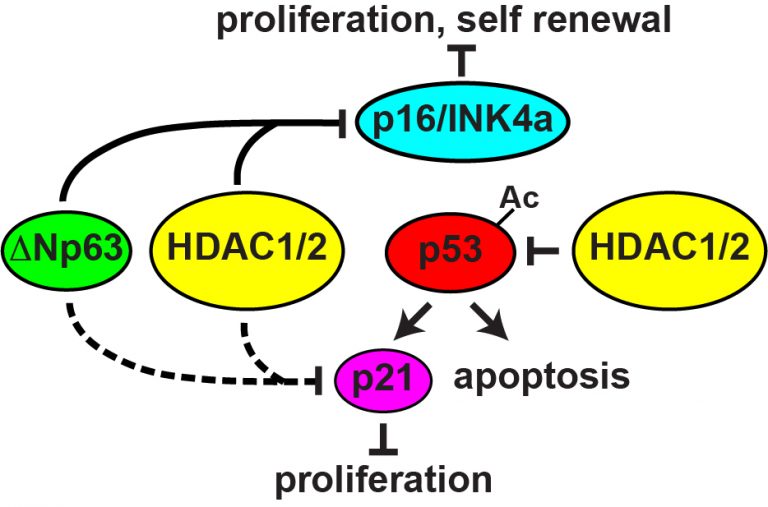
Model for the functions of HDAC1 and HDAC2 in maintaining proliferation, self-renewal, and survival of epidermal stem cells.
4: Identifying pioneer transcription factors that control development and stem cell activity in skin and oral epithelia.
Regenerative processes in skin and oral epithelia require cross-talk of multiple cell signaling and epigenetic mechanisms; failure of this communication leads to a range of diseases from skin cancers to hair loss conditions. Delineating how these inputs are coordinated to impact gene expression at the level of chromatin has potential to identify novel therapies for skin dysfunction. Pioneer transcription factors initiate gene expression by binding nucleosome-enriched silent chromatin and recruiting chromatin modifiers to provide access for transcription machinery. Key regulatory genes in hair follicle stem cells are associated with “super enhancers” containing binding sites for multiple transcription factors, suggesting that transcription factors activated in response to diverse signals collaborate with each other and with chromatin modifiers to coordinate cell-type specific transcriptional output. We are using genetic mouse models to investigate the roles of several families of putative pioneer transcription factors in controlling skin and oral stem cell proliferation and differentiation.
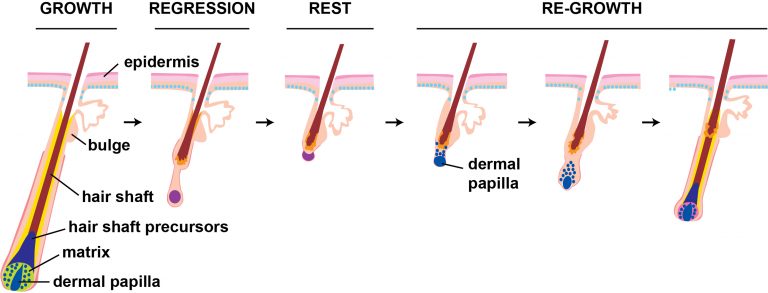
Hair follicles grow in cycles that are controlled by cell-cell signaling mechanisms and specific sets of transcription factors. Cells with active Wnt signaling are shown in blue, with deeper color indicating higher signaling levels.
5: Uncovering the cellular and molecular mechanisms underlying loss of taste and smell in COVID-19 disease.
Alterations of taste and smell appear early in COVID-19 disease progression, can occur in the absence of other symptoms, serve as a diagnostic tool for COVID-19, and can persist long-term in some patients. However, the basis for these defects in poorly understood. To address this question, we are using newly generated hACE2fl knockin mice that express hACE2 in similar cell types to humans, exhibit robust disease after infection with WT SARS-CoV-2, and allow us to test the cell type-specific requirements for symptoms associated with COVID-19 disease via tissue-specific Cre-mediated hACE2 deletion. We are using this powerful new tool to identify the oral, nasal and neuronal cell populations that are damaged by SARS-CoV-2 infection, and whose infection by SARS-CoV-2 leads to short- and long-term taste and smell defects. These experiments have potential to reveal new preventative and/or therapeutic targets in COVID-19 disease and shed insight into long-term as well as acute human pathologies.
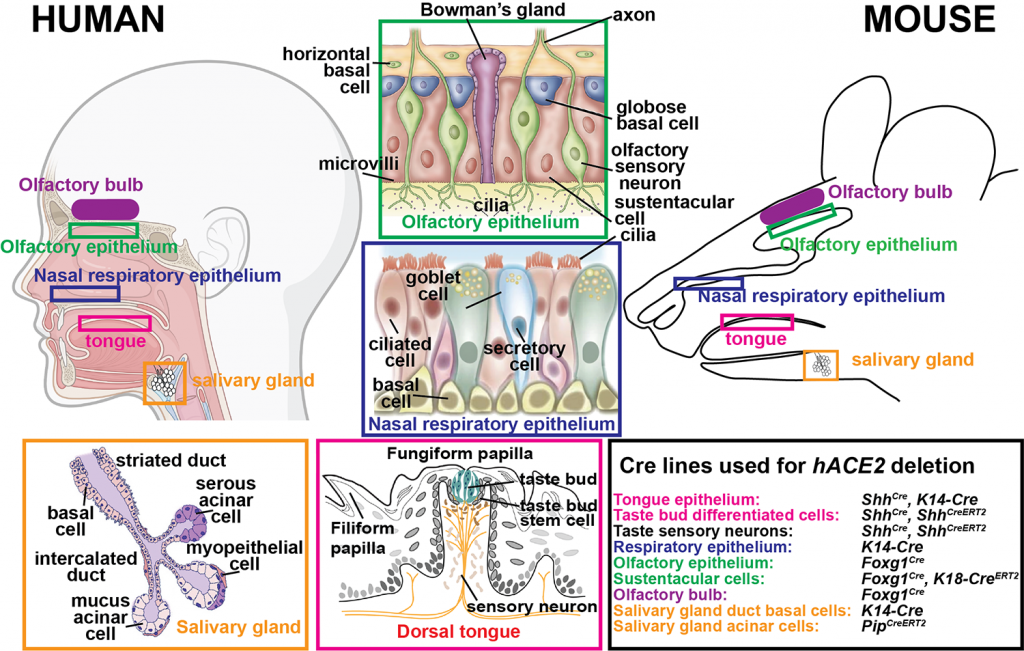
Uncovering the cellular and molecular mechanisms underlying loss of taste and smell in COVID-19 disease.
Sarah E. Millar, Ph.D.
Director, Black Family Stem Cell Institute
Professor, Departments of Cell, Developmental and Regenerative Biology and Dermatology
Icahn School of Medicine at Mount Sinai
Icahn Building, Floor 13 Room 020C
1425 Madison Ave
New York, NY 10029
Tel: (212) 659-9412
Email: sarah.millar@mssm.edu
Nyomi Cepeda, Administrative Coordinator
Black Family Stem Cell Institute
Icahn School of Medicine at Mount Sinai
Icahn Building, Floor 13 Room 79, Box 1496
1425 Madison Ave
New York, N.Y. 10029
Tel: 212-659-6897 (ext: 86897)
Cell: 646-581-8011
nyomi.cepeda2@mssm.edu
Kayrin Velez, Administrative Assistant
Black Family Stem Cell Institute
Icahn School of Medicine at Mount Sinai
Icahn Building, Floor 13 Room 70, Box 1496
1425 Madison Ave
New York, N.Y. 10029
Tel: 212-659-8131 (ext: 88131)
kayrin.velez@mssm.edu
