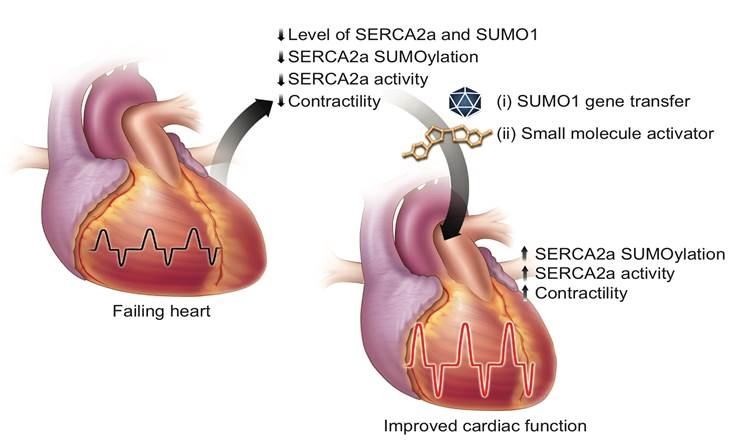
We have been focusing on identifying and characterizing novel molecular pathways with relevance to human disease. One area of particular interest to us is the role of post-translational modification of key proteins involved in physiological dysfunction in the heart. We developed a number of methodologies for building dynamic protein-protein interaction maps and identified important lysine post-translational modifications of the sarcoplasmic reticulum calcium ATPase (SERCA2a) pump, a key enzyme for muscle relaxation.
We demonstrated that small ubiquitin-related modifier type 1 (SUMO-1)-mediated modification is a critical post-translational modification that positively regulates SERCA2a function (Kho et al., Nature, 2011). Both murine and swine model studies suggest that increasing SUMO-1 levels may be a new therapeutic strategy to treat heart failure. Furthermore, the potential of this strategy is reinforced by the fact that the novel SUMO activator, N106, is a first-in-class agent with significant effects on myocardial dysfunction (Kho et al., Nature Communications, 2015). We use both in vitro proteomic approaches, biochemistry and in vivo mouse physiology analyses. Current research projects of my group as follows:
- Identifying SUMO-1 modified proteome under the physiological and pathological conditions and also identifying new targets for preventive and therapeutic purposes for heart failure.
- Understanding the regulation of SUMO-1 gene by microRNAs in the heart. We are focused now on determining whether microRNA-mediated SUMO-1 regulation would be beneficial in cardiac dysfunction.
- Characterizaing the relationship of post-translational acetylation of SERCA2a in the myocardium and their potential as new molecular pathway unerpinning physiological dysfunction and heart failure.
This research aims to validate the SUMO pathway and its potential therapeutic target for heart failure.
