Welcome!
The Fang Laboratory resides within the Department of Orthopaedics at Icahn School of Medicine at Mount Sinai. Our research program involves biomechanics, development biology, and tissue engineering. A major focus is the mechanistic understanding of regulatory factors (i.e., mechanical cues, transcription factors) maintaining cell identity and driving cell lineage commitment during musculoskeletal tissue development, mechanical adaptation, and pathogenesis.
Selected Publications
2022
Fang F, Xiao Y, Zelzer E, Leong KW, Thomopoulos S. A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell 2022 Dec;29(12): 1669-1684. PMID:36459968 (Commented by the Preview: Craft A, Gallowway J. Specialized cells for building tissue bridges Cell Stem Cell 2022)
Fang F, Linstadt RT, Genin GM, Ahn K, and Thomopoulos S. Mechanically competent chitosan‐based bioadhesive for tendon‐to‐bone repair. Adv Healthc Mater 2022 May;11(10). PMCID: PMC9117437.
Abraham A, Fang F, Golman M, Oikonomou P, Thomopoulos S. The role of loading in murine models of rotator cuff disease. J Orthop Res 2022 Apr;40(4):977-986. PMCID: PMC8639623.
Fang F, Sup M, Luzzi A, Ferrer X, Thomopoulos S. Hedgehog signaling underlying tendon and enthesis development and pathology. Matrix Biol 2022 Jan;105:87-103. PMCID: PMC8821161.
2020 & Previous
Fang F, Schwartz AG, Moore ER, Sup ME, Thomopoulos S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci Adv 2020 Oct 30;6(44). PMCID: PMC7608799.
Fang F, Lake SP. Multiscale mechanical evaluation of human supraspinatus tendon under shear loading after glycosaminoglycan reduction. J Biomech Eng 2017 Jul 1;139(7):0710131-0710138. PMCID: PMC6993773.
Fang F, Lake SP. Multiscale mechanical integrity of human supraspinatus tendon in shear after elastin depletion. J Mech Behav Biomed Mater 2016 Oct;63:443-455. PMID: 27472774.
Fang F, Lake SP. Modeling approaches for evaluating multiscale tendon mechanics. Interface Focus 2016 Feb 6;6(1):20150044. PMCID: PMC4686236.
Fang F, Sawhney A, Lake SP. Different regions of bovine deep digital flexor tendon exhibit distinct elastic, but not viscous, mechanical properties under both compression and shear loading. J Biomech 2014 Sep 22;47:2869-2877. PMID: 25113805.
Fang F, Lake SP. Multiscale strain analysis of tendon subjected to shear and compression demonstrates strain attenuation, fiber sliding, and reorganization. J Orthop Res 2015 Nov;33(11):1704-12. PMID: 26036894.
Research Overview
Our lab aims to better understand mechanobiology, cell biology, and development biology to pave the way for developing tissue engineering strategies and cell-based therapies to regenerate and rejuvenate musculoskeletal tissues. Our ongoing projects are in the following areas.
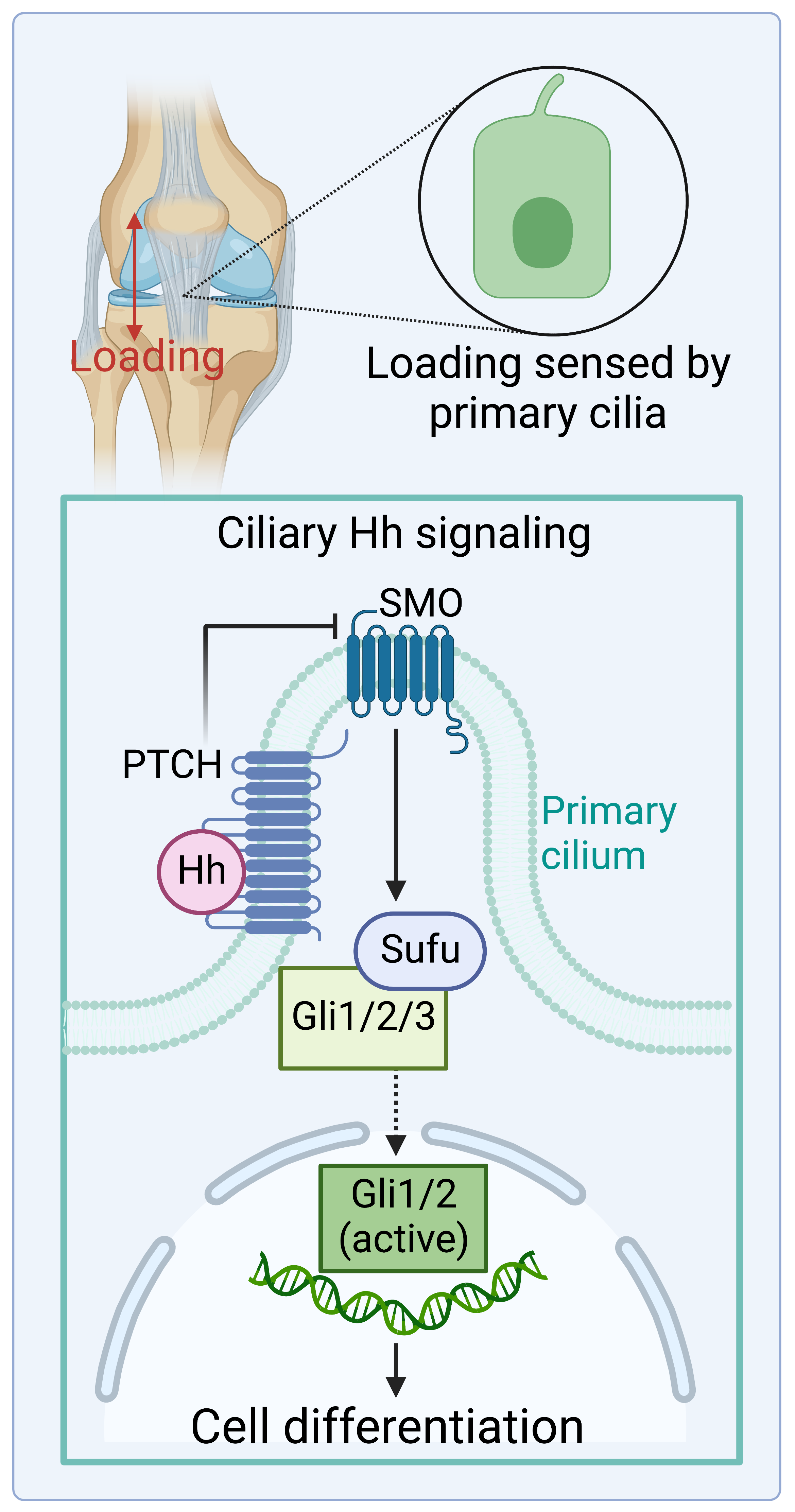
Tendon & Enthesis Mechanotransduction via Primary Cilia
Tendons are mechanosensitive load-bearing tissues. Decreased loading causes defects during development and structural remodeling in adult tendons. To further illustrate underlying principles, tendon progenitor/stem cells have been reported to acquire chondrogenic and osteogenic features after mechanical stimulation. Besides physical loading, hedgehog (Hh) signaling has been demonstrated to induce tenogenic and chondrogenic differentiation of stem cells. However, our understanding of the cellular mechanisms of tendon cell fate decisions regulated by physical loading is still in its infancy. Our recent work led to the primary cilium, an underappreciated subcellular membrane-covered protrusion emanating from the apical surface of mammalian cells. Primary cilia have been demonstrated to receive, transduce, and coordinate a vast variety of signals, including mechanical cues and Hh signaling, to mediate cell proliferation and differentiation for tissue function. This project aims to gain a though information of how tendon and enthesis cells interpret mechanical stimuli and Hh signaling via primary cilia to initiate tissue formation and regeneration.
Cells expressing Gli1, a transcription factor indicative of Hh activation, are the primary population that builds the tendon enthesis and facilitates the formation of a mineral graded fibrocartilage. Gli1-responsive (Gli1+) cells also act as stem/progenitor cells to maintain tissue homeostasis and regulate tissue regeneration or pathological tissue formation in meniscus, bone, bone marrow, tooth, and colon. However, how the stemness of these Gli1+ cells is maintained, and how their tissue- and context-specific nature is controlled, remains a mystery. Our results indicate that Gli1+ cells have enormous potential for cell therapies against diseases and injuries of musculoskeletal tissues (i.e., tendon, meniscus). Therefore, this project will evaluate how Gli1+ stem cell niches heavily shape their destiny and function in healthy and diseased tissues.
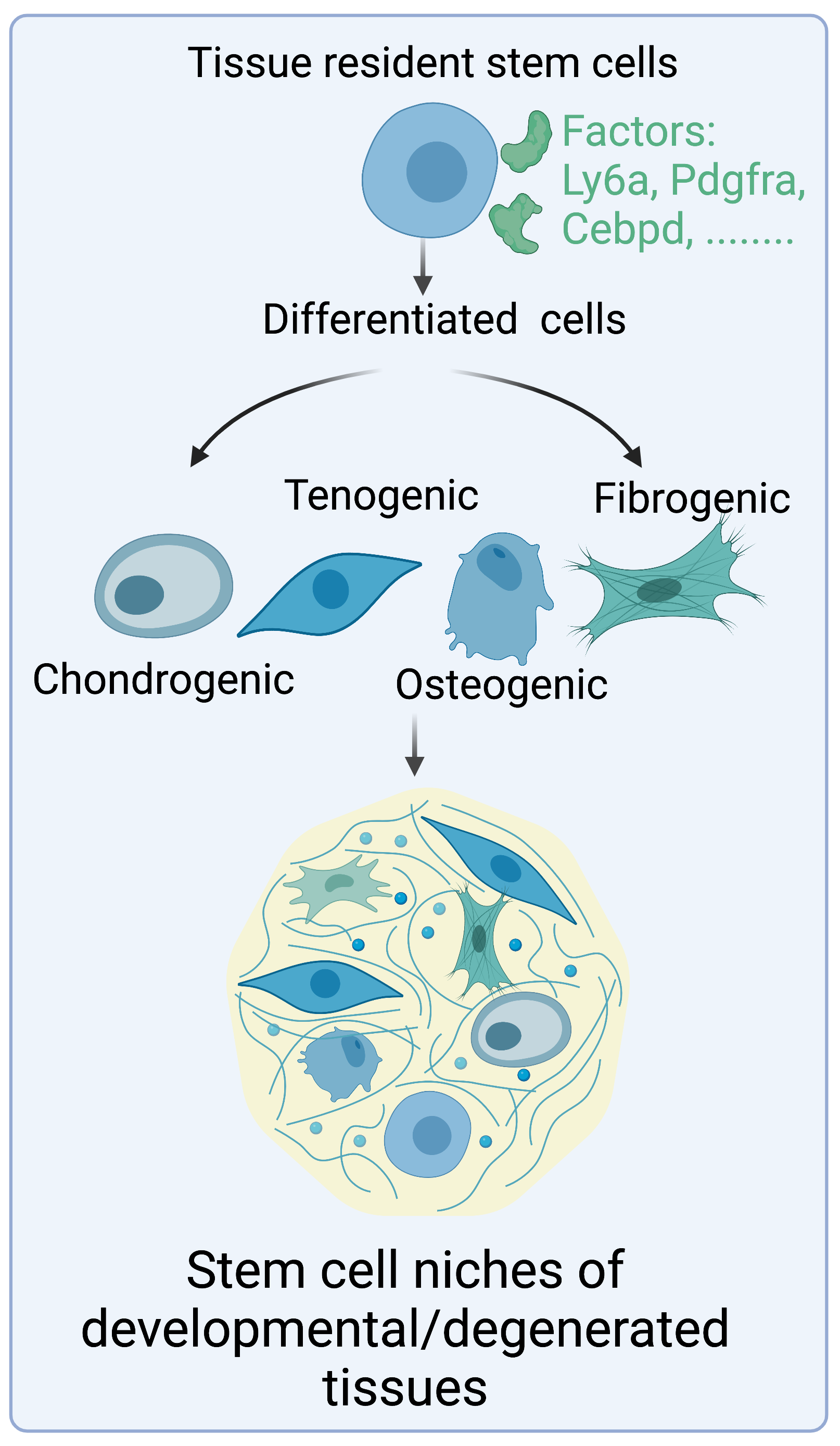
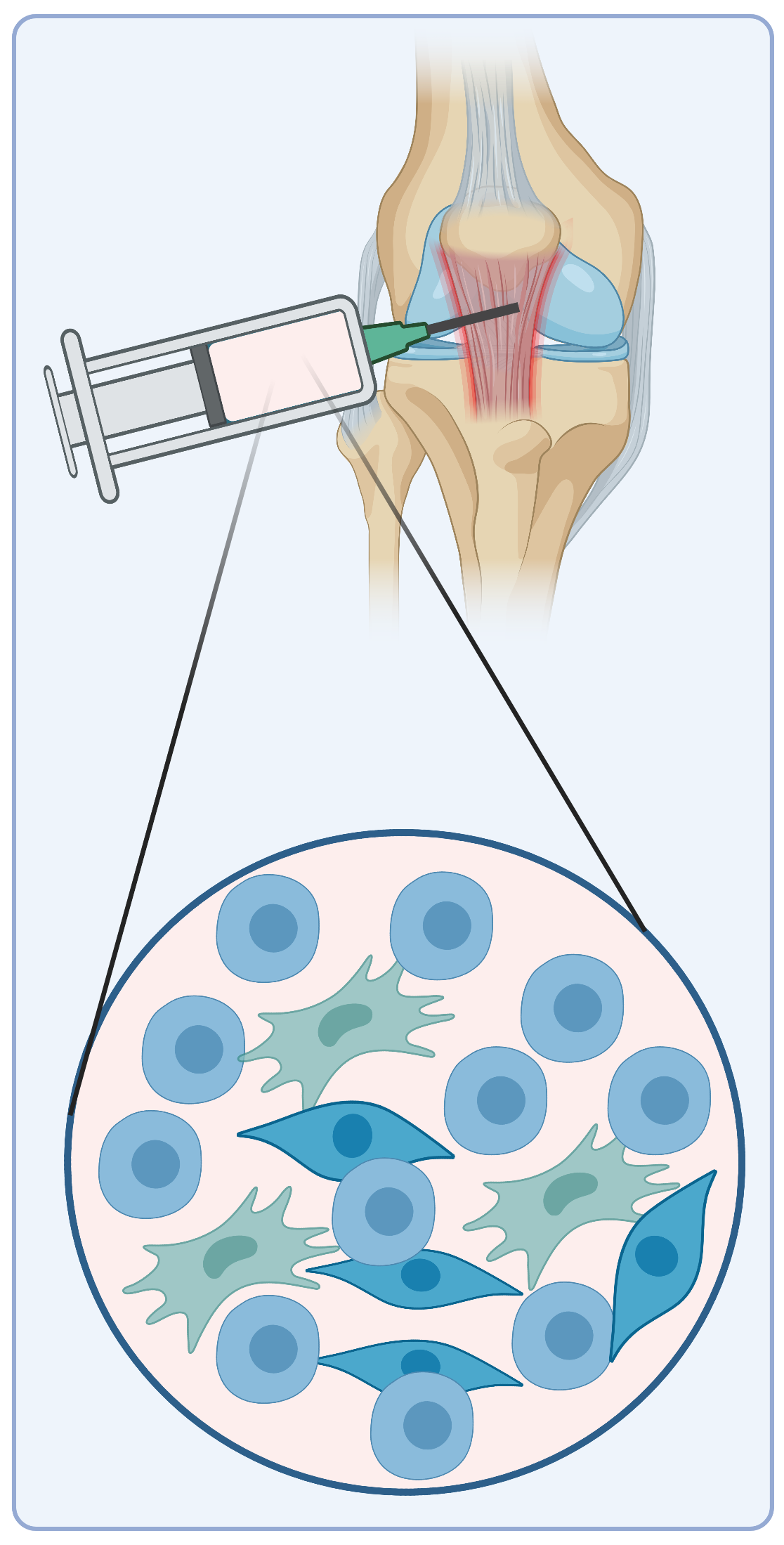
One of the causes contributing to poor healing of aged musculoskeletal tissues is that mature tissues have decreased stem cell numbers and activities. To overcome this hurdle, cell-based therapies have been proposed as a strategy to trigger native tissue formation and function restoration. Since tissue-derived stem cells have lineage-specific differentiation, they present as a potentially attractive cellular source. These stem cells might also easily integrate with host tissues. Using scRNA-seq and in vitro pluripotency assays, we have found that Gli1+ cells in the tendon enthesis exhibited clonogenicity and multipotency and these cells can significantly improve enthesis healing. Therefore, we aim to develop protocols to prepare the repertoire of Gli1+ stem cells for tendon and enthesis regeneration.
Team


Andryus Planutis received a BS in Biochemistry and Cell Biology from the University of California San Diego, and a medical degree (MB BCh BAO) from University College Dublin in Ireland. In the lab of Dr. Randall Holcombe, he explored the Wnt signaling pathway’s involvement in colon cancer. His work focused on the effects of Wnt pathway inhibition on cell morphology and colony formation, as well as the role of the stem cell marker Lgr5 in chemotherapy sensitivity. Currently, as a member of the Fang lab, Andryus is studying the mineralization of tendon enthesis, and hopes to discover how to enhance tendon healing after injury by modulating this mineralization process. Outside the lab, Andryus enjoys running, reading, and gardening.

Angel Moonilall graduated from John Jay College with a B.S. in Forensic Science. She now focuses her master’s research on studying enthesis development in human fetal and cadaver tissue, seeking to elucidate the developmental pathways and molecular mechanisms involved in enthesis formation across different stages of life. With a keen focus on identifying the similarities between mice and humans in this field, her work holds promise for advancing our understanding of musculoskeletal development and may offer insights into potential strategies for enhancing tissue regeneration and injury repair. In her free time, Angel enjoys watching anime, lifting weights, and being with family.

Julia Robbins received her B.A. in Biochemistry from Barnard College of Columbia University in 2021. During her undergraduate years, Julia worked as a research assistant within Dr. Subhasish Chatterjee’s lab, studying the self-assembly and co-assembly of small peptides in the presence of various co-solvents or electric fields of varying strengths using Molecular Dynamic (MD) simulations. As a master’s student in Dr. Fei Fang’s Lab, Julia hopes to learn more about tendon enthesis formation and its possible connection to heterotopic ossification. Julia enjoys playing the violin, playing sports, such as lacrosse and golf, and spending quality time with her family.

Matthew Casserly graduated from Worcester Polytechnic Institute with a B.S. in Biomedical Engineering. He worked in Professor Glenn Gaudette’s lab throughout his undergraduate studies independently examining plants as scaffolds for heart regeneration using thick tissue from gelling media. While working in Professor Fang’s Lab, he hopes to learn more about the chemicals and drugs that help target primary cilia and the effects it can have on tendons and ligaments over time. Additionally, Matthew hopes to gain further lab experience with biology and tissue engineering to prepare for graduate school and research all together. Aside from research, he enjoys bowling and traveling with friends and family at different restaurants.
Saswati Kar obtained her BSc in Systems Biology from the University of Hyderabad, India and her MS in Biology from the Indian Institute of Science Education and Research (IISER) in Pune, India. During her masters research in the lab of Prof Kundan Sengupta, she studied the role of nuclear lamins and nucleoporins in regulating early neuronal differentiation. As a current PhD student in the Fang Lab, Saswati aims to understand cell fate changes involved in the formation of enthesis fibrocartilage cells and their regenerative potential. When not at work, Saswati is found making digital art, learning new languages, catching up on her favorite TV shows, and on a particularly pleasant day, might be found strolling in the Central Park.
Yehuda Masturov is a second year medical student in the CUNY School of Medicine. He graduated from City College with a BS in biomedical science as part of the seven-year Sophie Davis Biomedical Program, which is a program that allows high school students to gain direct entry into medical school without taking the MCAT. He plans to pursue a career in orthopedic surgery and has shadowed several orthopedic surgeons in addition to participating in clinical research. As part of Dr. Fang’s lab, Yehuda is curious about tendon enthesis regeneration in the setting of an injury model. He plans to gain experience by coordinating and conducting mouse surgeries. In his free time, Yehuda enjoys lifting weights, playing sports, and spending time with his family.
Principal Investigator
Fei Fang, Ph.D.
Assistant Professor
The Leni & Peter W. May Department of Orthopaedics
The Icahn School of Medicine at Mount Sinai
Phone: 212-241-7041
Email: fei.fang@mssm.edu
Annenberg Building 20th, 20-72A
1456 Madison Avenue, New York, NY, 10029
https://icahn.mssm.edu/profiles/fei-fang
Photo credit: Yang Xiao (https://www.xiaoyang.nyc)
Get Involved
We are always looking for outstanding and motivated individuals to join our team. For postdocs, students, and technicians interested in research opportunities, please send an email with your CV to Dr. Fang (fei.fang@mssm.edu).
Postdoctoral Fellow
We invites enthusiastic and talented postdoctoral fellow with expertise in bioengineering, mechanobiology, tissue engineering, musculoskeletal tissues, and stem cells to apply.
Graduate Students
We have multiple positions and projects available for master students and doctoral rotation students. We are looking for interested students to join our laboratory.
The Leni & Peter W. May Department of Orthopaedics
The Icahn School of Medicine at Mount Sinai
Phone: 212-241-7041
Annenberg Building 20th, 20-72A
1456 Madison Avenue, New York, NY, 10029



