| The physics of influenza virus transmission
| Antiviral resistance and influenza virus fitness
_________________________________________________________________
THE PHYSICS OF INFLUENZA VIRUS TRANSMISSION
—
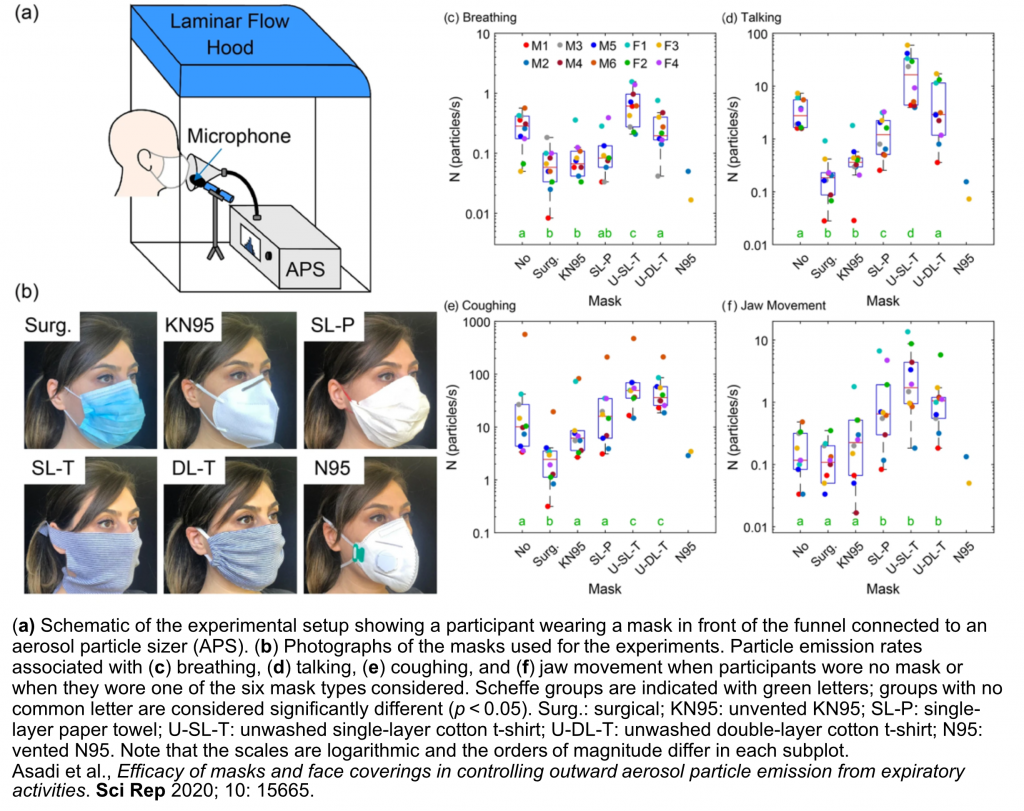
Precisely how influenza spreads among humans has long been a matter of debate. Human-to-human transmission of respiratory viruses is likely to occur by multiple routes, including contact (by direct touch between infected and susceptible persons, or by indirect touch of virus-contaminated surfaces, called “fomites,” by the susceptible person, who then self-inoculates by touching nose, mouth, or eyes) and non-contact or airborne routes (by ballistic droplet sprays impacting directly in the respiratory tract of a susceptible person, or by microscopic aerosol particles tiny enough to remain suspended in the air for minutes to hours until they are inhaled by a susceptible person); however, the relative contribution of each of these routes to influenza virus spread among humans remains unknown, and the physical composition and behavior of the infectious airborne particles passing between infected and susceptible hosts is just beginning to be elucidated. In collaboration with engineers William D. Ristenpart and Anthony S. Wexler in the College of Engineering at UC Davis, we are taking an interdisciplinary approach to studying the effect of host factors (like the emission and “superemission” of aerosol particles from the respiratory tract); environmental factors (such as airflow speed and the production of virus-contaminated environmental aerosol particles); and non-pharmaceutical interventions to prevent transmission (like masks and ventilation) on influenza virus transmission in humans and in animal models.
Asadi S, Tupas MJ, Barre RS, Wexler AS, Bouvier NM, Ristenpart WD. Non-respiratory particles emitted by guinea pigs in airborne disease transmission experiments. Scientific Reports 2021; 11(1): 17490.
Cappa CD, Asadi S, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Scientific Reports 2021; 11(1): 12110.
Asadi S, Cappa CD, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Scientific Reports 2020; 10(1): 15665.
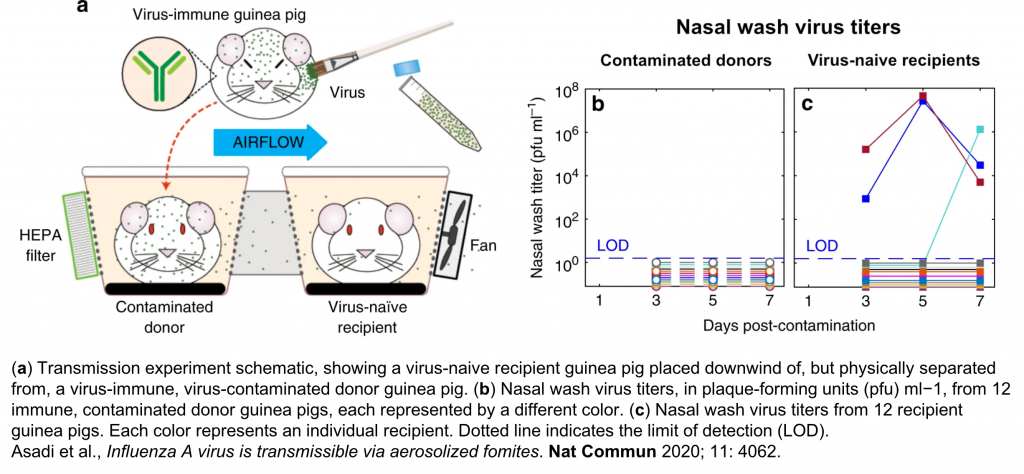
Asadi S, Gaaloul Ben Hnia N, Barre RS, Wexler AS, Ristenpart WD, Bouvier NM. Influenza A virus is transmissible via aerosolized fomites. Nature Communications. 2020; 11(1): 4062.
Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Effect of voicing and articulation manner on aerosol particle emission during human speech. PLoS One 2020 Jan 27; 15(1): e0227699.
Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Scientific Reports 2019; 9: 2348.
_________________________________________________________________
ANTIVIRAL RESISTANCE AND INFLUENZA VIRUS FITNESS
—
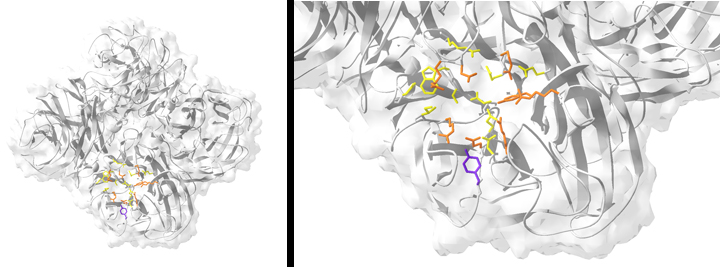
FIGURE: Resistance to the neuraminidase (NA) inhibitor oseltamivir is conferred by a single point mutation in the NA enzyme active site. (Left) Ribbon structure of the tetrameric NA from the oseltamivir-resistant clinical isolate influenza A/New York/08-1326/2008 (H1N1). (Right) The active site of NY/08-1326. Catalytic residues are colored orange, and framework residues are colored yellow. The resistance mutation H275Y is highlighted in purple.
—
Into the early 2000s, it was assumed that neuraminidase inhibitor (NAI)-resistant influenza viruses would be clinically irrelevant; amino acid mutations conferring NAI resistance were thought to be too costly to viral fitness. This prediction was proved wrong between 2007 and 2009, when virtually all seasonal influenza A(H1N1) (“sH1N1”) viruses, typified by the vaccine strain A/Brisbane/59/2007 (H1N1) (“Bris/59”), became resistant to the neuraminidase (NA) inhibitor oseltamivir.
We hypothesized that the oseltamivir-resistant NA conferred a fitness advantage — specifically, more efficient transmissibility — upon Bris/59-like sH1N1 viruses, thus explaining the rapidity with which viruses encoding an oseltamivir-resistant NA replaced earlier strains encoding a sensitive NA.
We performed a series of transmission experiments in the guinea pig model with the Bris/59-like clinical isolates A/NY/08-1253/2008 (H1N1) (oseltamivir-sensitive), A/NY/08-1326/2008 (H1N1) (oseltamivir-resistant), and reverse genetics- (rg-) derived reassortants of the two.
—
FIGURE: Guinea pig transmission of A/Brisbane/59/2007-like seasonal influenza A(H1N1) viruses is enhanced by expression of an oseltamivir-resistant neuraminidase. The label “(R)” indicates viruses with an oseltamivir-resistant NA, and “(S)” indicates viruses with an oseltamivir-sensitive NA. **, p < 0.01. by the log-rank test.
—-
A Kaplan-Meier time-to-event analysis of data reported previously [Bouvier et al., Journal of Virology 2012 Jul; 86(13): 7268-79] demonstrates that, collectively, Bris/59-like viruses encoding an oseltamivir-resistant NA — including an rg-derived 7:1 reassortant encoding the resistant NA, expressed in the context of the remaining seven segments from the oseltamivir-sensitive isolate, and a 6:2 reassortant encoding both the hemagglutinin (HA) and the NA from the resistant virus, in a backbone of the sensitive isolate — transmitted significantly more rapidly than did viruses encoding the oseltamivir-sensitive Bris/59 NA or a Bris/59-like NA.
Overall, with this research and prior work, we have demonstrated that “viral fitness” is a polygenic trait, and context-dependent compensatory mutations can evolve to restore efficient mammalian transmissibility to oseltamivir-resistant viruses.
Hai R, Schmolke M, Leyva-Grado VH, Thangavel RR, Margine I, Jaffe EL, Krammer F, Solórzano A, García-Sastre A, Palese P, Bouvier NM. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nature Communications 2013 Dec 10; 4: 2854.
Bouvier NM, Rahmat S, Pica N. Enhanced mammalian transmissibility of seasonal Influenza A/H1N1 viruses encoding an oseltamivir-resistant neuraminidase. Journal of Virology 2012 Jul; 86(13): 7268-79.
Seibert CW, Rahmat S, Krammer F, Palese P, Bouvier NM. Efficient transmission of pandemic H1N1 influenza viruses with high-level oseltamivir resistance. Journal of Virology 2012 May; 86(9): 5386-9.
Bouvier NM, Lowen AC, Palese P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. Journal of Virology 2008 Oct; 82(20):10052-8.
_________________________________________________________________