Arrhythmia mechanisms and Therapies in heart failure
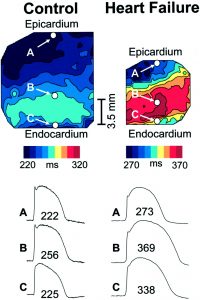
Over the past 17 years, we have developed a major interest in unraveling the mechanisms of sudden cardiac death in heart failure. We demonstrated the .role of repolarization heterogeneity and conduction abnormalities in promoting arrhythmias across the failing heart. Our work highlighted the importance of gap junction remodeling including altered phosphorylation of Cx43 in the conduction defects that we uncovered in small and large animals of heart failure. We also identified the time-course of electrophysiological changes during the early stages of hypertrophy. We studied the mechanisms by which mechanical dyssynchrony and cardiac resynchronization therapy regulate electrophysiological function at the cellular and tissue levels.
- Akar FG, Rosenbaum DS. Transmural Electrophysiological Heterogeneities Underlying Arrhythmogenesis in Heart Failure. Circ Res. 2003; 93(7):638-45.
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF (2004). Mechanisms Underlying Conduction Slowing and Arrhythmogenesis in Non-ischemic Cardiomyopathy. Circ Res. 2004; 95(7): 717-25.
- Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic Changes in Conduction Velocity and Gap Junction Properties During Development of Pacing Induced Heart Failure. Am J Physiol Heart Circ Physiol. 2007; 293(2): H1223-30.
- Jin H, Chemaly ER, Lee A, Kho C, Hadri L, Hajjar RJ, Akar FG (2010). Mechano-electrical remodeling and arrhythmias during progression of hypertrophy. FASEB J. 2010; 24(2):451-63.
Metabolic regulation of arrhythmogenesis
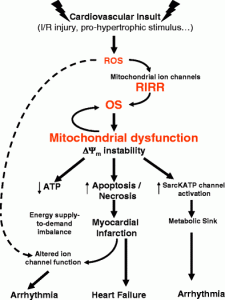
A major focus of my lab has been on the identification of mechanisms by which mitochondrial bioenergetics control electrical function. We were among the first groups to highlight the central role of mitochondria in the regulation of excitability and arrhythmias through a mechanism we termed ‘metabolic sink’. We further identified novel strategies for treating post-ischemic arrhythmias by targeting key mitochondrial ion channels.
- Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005; 115(12):3527-35. Epub 2005. PubMed PMID: 16284648; PubMed Central PMCID: PMC1280968.
- Xie C, Kauffman J, Akar FG. Functional crosstalk between the mitochondrial PTP and KATP channels determine arrhythmic vulnerability to oxidative stress. Front Physiol. 2014; 5:264. doi: 10.3389/fphys.2014.00264. eCollection 2014. PMID: 25076913; PMCID: PMC4099963.
- Lyon AR, Joudrey PJ, Jin D, Nass RD, Aon MA, O’Rourke B, Akar FG. Optical imaging of mitochondrial function uncovers actively propagating waves of mitochondrial membrane potential collapse across intact heart. J Mol Cell Cardiol. 2010 Oct; 49 (4):565-75. doi: 10.1016/j.yjmcc.2010.07.002. Epub 2010 Jul 16. PubMed PMID: 20624394; PubMed Central PMCID: PMC3081287.
- Kim GE, Ross JL, Xie C, Su KN, Zaha VG, Wu X, Palmeri M, Ashraf M, Akar JG, Russell KS, Akar FG, Young LH. LKB1 deletion causes early changes in atrial channel expression and electrophysiology prior to atrial fibrillation. Cardiovasc Res. 2015; 108(1):197-208. PMID: 26378152.
Arrhythmia mechanisms in diabetes mellitus
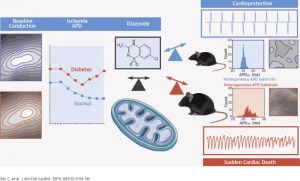
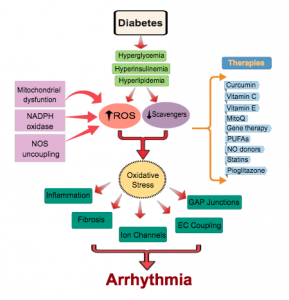
Recently, we have been studying the mechanisms that underlie electrical dysfunction in the setting of diabetes mellitus. We identified the arrhythmogenic role of reactive oxygen species (ROS) secondary to GSH oxidation. We are working towards identifying which mitochondrial pathways are protective and which are defective in diabetes. We are also testing novel approaches for arrhythmia suppression in the diabetic heart by targeting the mitochondrial translocator protein which controls the regenerative process of ROS-induced ROS-release.
- Xie C, Hu J, Motloch LJ, Karam BS, Akar FG. The classically cardioprotective agent diazoxide elicits arrhythmias in type-2 diabetes mellitus. J Am Coll Cardiol 2015; 66(10):1144-56.
- Xie C, Biary N, Tocchetti CG, Aon MA, Paolocci N, Kauffman J, Akar FG. Glutathione oxidation unmasks proarrhythmic vulnerability of chronically hyperglycemic guinea pigs. Am J Physiol Heart Circ Physiol. 2013; 304(7):H916-26.
- Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O’Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes. 2012; 61(12):3094-105.