I. Neurosensory cell specification, differentiation and maintenance and regeneration
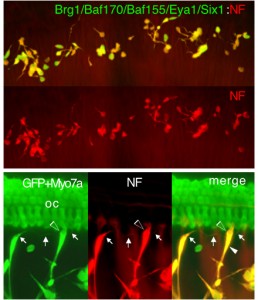
The mammalian inner ear uses sensory cells (the hair cells) for mechanoelectric transduction of vestibular and auditory stimuli and conducts this information to the brain via sensory neurons. In mammals, lost hair cells and sensory neurons are not spontaneously replaced and result in irreversible deafness, which is one of the most widespread and poorly understood disabilities in the world. It is currently unknown why the mammalian cochlea, which retains supporting cells, cannot replace lost hair cells. We have shown reported that Eya1 and Six1 form a transcriptional complex and interact with Sox2 to activate specific developmental program controlling hair cell fate induction. Misexpression of Eya1/Six1 with low dose of Sox2 is able to convert cochlear nonsensory cells to hair cells (Developmental Cell, 2012). In contrast, misexpression of Eya1/Six1 with the SWI/SNF chromatin regulators can induce transdifferentiation of cochlear nonneurosensory cells or mouse fibroblast cells to spiral ganglion neurons (Development, 2012). Given the desperate need for auditory repair strategies, these results stimulate further research into the development of new therapeutic strategies for auditory regeneration and repair.
- With funding from NYStem, we are currently exploring whether misexpression of the neurosensory cell-specific factors Eya1/Six1 in combination with different cofactors and chromatin regulators in damaged cochlea is sufficient to induce nonsensory cells to regenerate functional hair cells or neurons and whether fibroblast-derived neuronal cells converted by these factors are functional for neuronal repair in animal models.
- Chromatin remodelers are a group of epigenetic regulators that disrupt DNA-protein contacts and facilitate recruitment of essential factors required for transcription to regulate gene expression. With funding from NIH/NIDCD, we are characterizing how chromatin remodeling-protein and its interaction with Eya1 and its cofactors to regulate inner ear neurogenesis and sensory hair cell differentiation and maintenance.
II. Elucidate regulatory network controlling ureteric patterning and kidney development
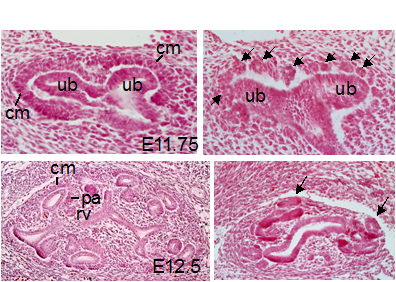
Congenital kidney and urinary tract disorders are the most common types of birth defects. The balance between self-renewal and differentiation of nephron progenitor/stem cells is essential for generation of sufficient number of nephrons in a mature kidney. We have found that the Eya and Six protein families are also critical regulators for renal development and mutations in these genes cause renal agenesis and hypoplasia as well as hydronephrosis. Recently, we reported that Six2 mediates nuclear translocation of Eya1, where Eya1 uses its threonine-phsophatase activity to control Myc phosphorylation and function in the nephron progenitors (Developmental Cell, 2014). This work revealed a functional link between Eya1, Six2 and Myc in driving the expansion and maintenance of the multipotent progenitor population during nephrogenesis. With funding from NIH/NIDDK, we are currently investigating the mechanisms by which EYA1 regulates expansion of metanephric mesenchymal stem/progenitor cell population and determining whether EYA1’s phosphatase activity is necessary for kidney development.
III. Characterize EYA1’s partner proteins
In an effort to identify binding partners for EYA1/SIX, we performed yeast two-hybrid screen and mass spectrometry analysis and have identified several interesting partner factors for EYA1. We are currently characterizing the functional significance of their interaction in vivo.