Biology of Adeno-Associated Virus
One area of interest of our lab is the biology of adeno-associated virus (AAV). Adeno-associated virus is a small, non-pathogenic virus that shows great promise as a vector for gene therapy applications. AAV is a non-enveloped virus of the family Parvoviridae with a single-stranded DNA genome and an icosahedral capsid composed of the structural proteins VP1, VP2, and VP3. A particularly attractive feature of AAV is that — at least in “postmitotic” tissues such as the liver and the heart — AAV transduction results in the persistent expression of the target gene even in the absence of genome integration.
Our lab is interested in understanding the cellular mechanisms that underlie the cell and tissue specificity of AAV. The different AAV serotypes can transduce several cell types and tissues, the precise nature of which depends on the specific serotype. These serotype-specific expression patterns can be explained only partially by variations in receptor densities in different tissues and cell types. In contrast, it is likely that other cellular barriers are playing a role in these differences. Our goal is to improve our understanding of the cellular roadblocks that prevent the efficient transduction of certain cell types.
Our recent work in this area has concentrated on two aspect of the infectious entry of AAV, namely the mechanism of endocytosis and the subsequent trafficking of AAV to the perinuclear area of the cell.
Mechanism of Endocytosis of AAV.
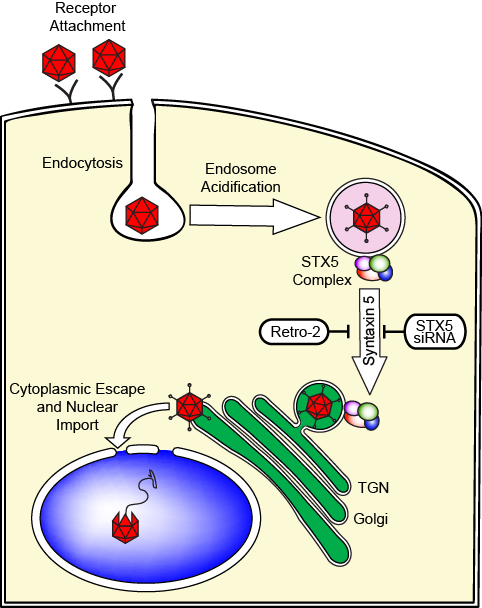
It has been reported in the past that AAV, at least serotype 2, is endocytosed via clathrin mediated endocytosis. However, our own research in this area has demonstrated that clathrin mediated endocytosis is likely not a major entry pathway for AAV that leads to successful transduction of a cell. Instead, we demonstrated that AAV is the first virus to be shown to enter the cell through the so-called CLIC/GEEC (Clathrin Independent Carrier/GPI-Anchored-Protein-Enriched Endosomal Compartment) pathway (Nonnenmacher and Weber, 2011). The CLIC/GEEC pathway is an as yet poorly understood endocytic entry mechanism that is used by several bacterial toxins, including cholera toxin, to enter the cell.
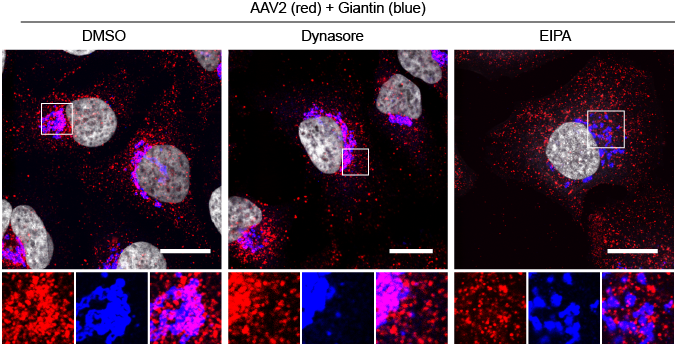
Interestingly, however, AAV can also enter the cells through a second pathway that is dependent on the GTPase dynamin, which is known to play a role in several endocytic mechanisms, including clathrin mediated endocytosis. Intriguingly, when the dynamin-dependent endocytosis of AAV is blocked by pharmacologically inhibiting dynamin activity with dynasore, AAV still can accumulate in the perinuclear region, where it accumulates in the absence of any drugs. On the other hand, when CLIC/GEEC mediated endocytosis is blocked with the amiloride analog EIPA, AAV remains in vesicles dispersed throughout the cytoplasm (Nonnenmacher and Weber, 2011).
These results and other data indicate that to be able to transduce a cell, AAV must reach the perinuclear region and, more specifically, the Golgi to transduce successfully a cells (Nonnenmacher and Weber, 2011).
Trafficking from the Cell Periphery to the Golgi
It has been proposed that AAV travels from the cell periphery to the perinuclear area through either the late endosomes (LE) or the recycling endosomes (RE). Our recent investigations, using a combination of siRNAs and small molecule inhibitors that target canonical LE and RE dependent pathways, showed that AAV uses a non-canonical endosome to Golgi pathway that depends on the tSNARE syntaxin 5 (Nonnenmacher et al., 2015)
Strikingly, siRNA-mediated knock-down of syntaxin 5 reduces transduction by most serotypes. Moreover, remarkably, Retro 2, an inhibitor of syntaxin 5 function, drastically reduces transduction of most cell types by most AAV serotypes (Nonnenmacher et al., 2015).
The fact that most, if not all, serotypes use the same endocytic mechanism and share the same trafficking pathway to the Golgi raises an obvious question: If receptor distribution does not fully explain tissue and cell type tropism and the endocytic and trafficking patterns are the same what, then, determines the tropism of the AAV serotypes?
We believe that a better understanding of the endocytic pathways and intracellular trafficking patterns used by AAVs will ultimately help us and others in designing novel AAV variants with specific cell and tissue tropism.
References