Molecular Mechanisms of Mammalian Fertilization
The goal of our research is to determine the molecular basis of:
- The different roles of mammalian egg zona pellucida (ZP) proteins ZP1-4 during oogenesis, fertilization, and early development, and
- The role of the zona pellucida domain (ZPD), a long conserved sequence found in ZP1-4 and many other proteins, in normal and diseased states.
 Research
Research
For all animals, maintenance of life and speciation depend on the process of fertilization. Fusion of eggs and sperm to form a zygote is the culmination of a complex series of interactions between two highly specialized gametes. In mammals, interactions between gametes begin when free-swimming sperm reach the site of ovulated eggs in the oviduct and then bind to the unfertilized egg’s extracellular coat, the zona pellucida (ZP), surrounding the plasma membrane. Identification of the ZP around human eggs dates back nearly 200 years.

Paul M. Wassarman
Department of Cell, Developmental, and Regenerative Biology
Icahn School of Medicine at Mount Sinai
Annenberg 25-30B, Box 1020
1468 Madison Avenue
New York, NY 10029-6574
Office: (212) 241-8616/8620
E-mail: paul.wassarman@mssm.edu
Current Projects
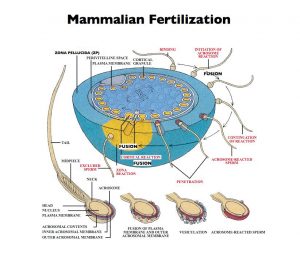
Our research has focused on mammalian egg and sperm surface components that account for species-restricted binding of sperm to eggs during fertilization. Among these components are proteins present in the ovulated egg’s ZP. In mice the unfertilized egg’s ZP consists of only three proteins called ZP1, ZP2, and ZP3. A fourth protein, ZP4, present in ZP of a number of other mammalian eggs, including human eggs, is very similar to ZP1. Over the years we were the first to (i) identify, name, and characterize ZP1-3; (ii) identify growing oocytes as the source of nascent ZP1-3; (iii) demonstrate that ZP2 and ZP3 are sperm receptors; (iv) demonstrate that ZP3 is an acrosome reaction- inducer; (v) determine the primary structure of ZP3; (vi) clone and characterize the gene encoding ZP3; (vii) identify cis-acting sequences and trans-acting factors that regulate ZP3 gene expression; and (viii) produce ZP3 heterozygous and homozygous null mice. While much of our research has been carried out with mice, ZP1-3 are present in the ZP of all mammalian eggs, including human eggs, and ZP1-3-like proteins are present as well in the extracellular coat of eggs from amphibians, birds, fish, and many invertebrates.
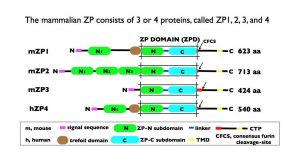
Schematic representation of the organization of mouse zona pellucida (ZP) proteins mZP1–3 and human ZP protein hZP4. In each case, the polypeptide has a signal sequence at the N terminus; a ZP domain (ZPD) consisting of ZP-N and ZP-C subdomains and a linker region; a consensus furin cleavage site (CFCS); transmembrane domain (TMD); and cytoplasmic tail in the C-terminal propeptide (CTP). mZP1 and hZP4 also have a trefoil domain adjacent to the ZPD. mZP2 [713 amino acids (aa)] has three additional ZP-N subdomains, N1–N3, between the N terminus of the polypeptide and the ZPD. mZP1 (623 aa) and hZP4 (540 aa) have one additional ZP-N subdomain, N1, between the N terminus of the polypeptide and the trefoil domain. mZP3 is the smallest of the three mouse ZP proteins (424 aa) and consists primarily of a ZPD. Notably, mZP1 and mZP2 have only three or four aa between the ZPD and the CFCS, whereas mZP3 has 47 aa, which include the sperm-combining site and represent a region of positive Darwinian selection during evolution.
Our studies revealed that ZP1-3 are synthesized, processed, and secreted by mouse oocytes, the precursors of eggs, during oogenesis. In mammals expression of single-copy genes encoding ZP1-3 is female specific. During growth of oocytes, nascent ZP1-3 are secreted, processed, and assemble using non-covalent interactions into an extensive network of interconnected fibrils that constitute the thick ZP matrix. The fibrils consist of ZP2-ZP3 dimers present every 140 Å or so along the fibrils, and the fibrils are interconnected by ZP1 and/or ZP4. Free-swimming sperm recognize and bind to a specific region of ZP3, the sperm combining-site. Binding to ZP3 activates the signal transduction pathway of sperm that culminates in exocytosis, the acrosome reaction, that enables bound sperm to penetrate the ZP and reach the egg’s plasma membrane. After undergoing the acrosome reaction, either spontaneously or due to binding to ZP3, acrosome-reacted sperm bind to ZP2. Therefore, ZP2 and ZP3 are structural proteins used to build a specialized extracellular matrix around eggs and are sperm receptors employed during fertilization. ZP3 is also an activator of signal transduction when acrosome-intact sperm bind to unfertilized eggs. Once eggs are fertilized by a single sperm ZP2 and ZP3 are modified and the structure of the ZP is altered such that additional free-swimming sperm can no longer bind to the ZP – part of the so-called block to polyspermy.
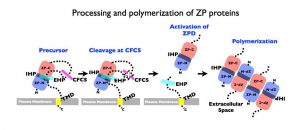
Schematic representation of a general mechanism for assembly of nascent ZP proteins. In all ZPD precursor proteins (Precursor), the ZPD (ZP-N and ZP-C) is followed by a C-terminal propeptide (CTP) that contains a basic cleavage site, such as a consensus furin cleavage-site (CFCS), and external hydrophobic patch (EHP), and a transmembrane domain (TMD) anchor site. Precursors do not polymerize within the cell either as a result of direct interaction between the EHP and internal hydrophobic patch (IHP) or because they adopt a conformation dependent on the presence of both hydrophobic patches. C-terminal processing at the CFCS by a proprotein convertase (Cleaved at CFCS, X) leads to dissociation of mature proteins from the EHP and activation of the ZPD (Activation of ZPD) for assembly (Polymerization) into fibrils and matrix.
All ZP proteins share a large region of polypeptide, approximately 270 amino acids in length, called the zona pellucida domain (ZPD). A ZPD has 8 conserved cysteine residues present as 4 intramolecular disulfides and arose more than 600 million years ago. A ZPD is also present in a wide variety of other proteins of diverse origins and functions. For example, ZPD-containing proteins are components of the mammalian egg, inner ear, nose, and kidney, as well as the Drosophila cuticle, wing epithelium, and mechanosensory organ. Our research strongly suggests that the ZPD is a conserved module used generally for polymerization of extracellular proteins. Since a ZPD is found in a number of proteins involved in human pathologies, including deafness, infertility, vascular and renal disease, and cancer, we are also interested in relationships between mutations in the ZPD and disease.
Publications
2020-2029
Wassarman, P.M. and Litscher, E.S.: Zona pellucida proteins, fibrils, and matrix. Annual Review Biochemistry 89: 695-715 (2020).
Wassarman, P.M.: A Place in History: The Biography of John C. Kendrew. Oxford University Press, 368 pp. (2020).
Litscher, E.S. and Wassarman, P.M.: Zona pellucida genes and proteins and human fertility. Trends Developmental Biology 13: 21-33 (2020).
Wassarman, P.M. and Litscher, E.S.: Zona pellucida genes and proteins: Essential players in mammalian oogenesis and fertility. Genes (Basel) 12: 1-22 (2021).
Wassarman, P.M. and Litscher, E.S.: Female fertility and the zona pellucida. eLife 11: e76106 (2022).
Wassarman, P.M. and Litscher, E.S.: Mammalian fertilization. In Brenner’s Encyclopedia of Genetics, 4th ed., in press (2023).
2010 - 2019
Plaza, S., Chanut-Delalande, H., Fernandes, I., Wassarman, P.M., and Payre, F.: From A to Z: Apical structures and zona pellucida-domain proteins. Trends Cell Biology 20: 524-532 (2010).
Wassarman, P.M. and Litscher, E.S.: Egg’s ZP3 structure speaks volumes. Cell 143: 337-338 (2010).
Wassarman, P.M. and Soriano, P.M. (Eds.): Guide to Techniques in Mouse Development, Part A, Mice, Embryos, and Cells. Methods in Enzymology 476: 539 pp. (2010) .
Wassarman, P.M. and Soriano, P.M. (Eds.): Guide to Techniques in Mouse Development, Part B, Mouse Molecular Genetics. Methods in Enzymology 477: 637 pp. (2010).
Wassarman, P.M.: The sperm’s sweet tooth. Science 233: 1708-1709 (2011).
Wassarman, P.M. and Litscher, E.S.: Influence of the zona pellucida of the mouse egg on folliculogenesis and fertility. International Journal Developmental Biology 56: 833-839 (2012).
Wassarman, P.M. and Litscher, E.S.: Biogenesis of the mouse egg’s extracellular coat, the zona pellucida. Current Topics in Developmental Biology 102: 243-266 (2013).
Wassarman, P.M. (Ed.): Gametogenesis. Current Topics in Developmental Biology 102: 434 pp. (2013).
Wassarman, P.M.: Reproductive biology: Sperm protein finds its mate. Nature 508: 466-467 (2014).
Litscher, E.S. and Wassarman, P.M.: Evolution, structure, and synthesis of vertebrate egg-coat proteins. Trends in Developmental Biology 8: 65-76 (2014).
Rosenwaks, Z. and Wassarman, P.M. (Eds.): Human Fertility: Methods and Protocols. Methods in Molecular Biology 1154: 584 pp. (2014).
Litscher, E.S. and Wassarman, P.M. (Eds.): A Guide to Zona Pellucida Domain Proteins. John Wiley and Sons, 208 pp. (2015).
Wassarman, P.M. (Ed.): Essays on Developmental Biology. Current Topics in Developmental Biology Part A 116: 744 pp.; Part B 117: 710 pp. (2016).
Wassarman P.M.: A personal perspective: My four encounters with John Kendrew. Journal Molecular Biology 429: 2594-2600 (2017).
Wassarman, P.M. and Litscher, E.S.: The mouse egg’s zona pellucida. Current Topics in Developmental Biology 130: 331-356 (2018).
Litscher, E.S. and Wassarman, P.M.: The fish egg’s zona pellucida. Current Topics in Developmental Biology 130: 275-306 (2018).
Litscher, E.S. and Wassarman, P.M. (Eds.): Extracellular Matrix and Egg Coats. Current Topics in Developmental Biology 130: 488 pp. (2018).
2000 - 2009
Wassarman, P.M., Jovine, L., and Litscher, E.S.: A profile of fertilization in mammals. Nature Cell Biology 3: E59-E64 (2001).
Qi, H., Williams, Z., and Wassarman, P.M.: Secretion and assembly of zona pellucida glycoproteins by growing mouse oocytes microinjected with epitope-tagged cDNAs for mZP2 and mZP3. Molecular Biology Cell 13: 530-541 (2002).
Jovine, L., Qi, H., Williams, Z., Litscher, E., and Wassarman, P.M.: The ZP domain is a conserved module for protein polymerization. Nature Cell Biology 4: 457-461 (2002).
Wassarman, P.M. and Keller, G.M. (Eds.): Differentiation of Embryonic Stem Cells. Methods in Enzymology 36: 535 pp. (2003) .
Jovine, L., Qi, H., Williams, Z., Litscher, E.S., and Wassarman, P.M.: A duplicated motif controls assembly of zona pellucida domain proteins. Proceedings National Academy Sciences, USA 101: 5922-5927 (2004).
Darie, C.C., Biniossek, M.L., Jovine, L., Litscher, E.S., and Wassarman, P.M.: Structural characterization of fish egg vitelline envelope proteins by mass spectrometry. Biochemistry 43: 7459-7478 (2004).
Jovine, L., Darie, C.C., Litscher, E.S., and Wassarman, P.M.: Zona pellucida domain proteins. Annual Review Biochemistry 74: 83-114 (2005).
Williams, Z., Litscher, E.S., Jovine, L., and Wassarman, P.M.: Polypeptide encoded by mouse ZP3 exon-7 is necessary and sufficient for sperm binding in vitro. Journal Cellular Physiology 207: 30-39 (2006).
Wassarman, P.M.: Fertilization: Welcome to the fold. Nature 456: 586-587 (2008).
Wassarman, P.M.: Zona pellucida glycoproteins. Journal Biological Chemistry 283: 24285-24289 (2008).
Wassarman, P.M. and Litscher, E.S.: Mammalian fertilization is dependent on multiple membrane fusion events. In, Cell Fusion: Methods and Protocols 475: 99-113, Methods in Molecular Biology (2008).
Wassarman, P.M. and Vacquier, V.D. (Eds.): Fertilization. International Journal Developmental Biology 52: 388 pp. (2008).
1990 - 1999
Lira, S.A., Kinloch, R.A., Mortillo, S., and Wassarman, P.M.: An upstream region of the mouse ZP3 gene directs expression of firefly luciferase specifically to growing oocytes in transgenic mice. Proceedings National Academy of Sciences, USA 87: 7215-7219 (1990).
Wassarman, P.M.: Profile of a mammalian sperm receptor. Development 108: 1-19 (1990).
Mortillo, S. and Wassarman, P.M.: Differential binding of gold-labeled zona pellucida glycoproteins mZP2 and mZP3 to mouse sperm membrane compartments. Development 113: 141-149 (1991).
Wassarman, P.M. (Ed.): Elements of Mammalian Fertilization, Vol. 1, Basic Concepts, Vol. 2, Practical Applications, 493 pp. (1991).
Kinloch, R.A., Mortillo, S., Stewart, C.L., and Wassarman, P.M.: Embryonal carcinoma cells transfected with ZP3 genes differentially glycosylate similar polypeptides and secrete active mouse sperm receptor. Journal Cell Biology 115: 655-664 (1991).
Wassarman, P.M. and DePamphilis, M.L. (Eds.): Guide to Techniques in Mouse Development. Methods in Enzymology 225: 1021 pp. (1993).
Wassarman, P.M.: Towards molecular mechanisms for gamete adhesion and fusion during mammalian fertilization. Current Opinion Cell Biology 7: 658-664 (1995).
Litscher, E.S, Juntunen, K., Seppo, A., Niemalä, R., Penttilä, L., Renkonen, O., and Wassarman, P.M.: Oligosaccharide constructs with defined structures that inhibit binding of mouse sperm to unfertilized eggs in vitro. Biochemistry 34: 4662-4669 (1995).
Liu, C., Litscher, E.S., Mortillo, S., Sakai, Y., Kinloch, R.A., Stewart, C.L., and Wassarman, P.M.: Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proceedings National Academy of Sciences, USA 93: 5431-5436 (1996).
Wassarman, P.M.: Mammalian fertilization: Molecular aspects of gamete adhesion, exocytosis, and fusion. Cell 96: 175-183 (1999).
Wassarman, P.M. and Wolffe, A.P. (Eds.): Chromatin. Methods in Enzymology 304: 815 pp. (1999).
Litscher, E.S., Qi, H., and Wassarman, P.M.: Mouse zona pellucida glycoproteins mZP2 and mZP3 undergo carboxy-terminal proteolytic processing in growing oocytes. Biochemistry 38: 12280-12287 (1999).
1980 - 1989
DePamphilis, M.L. and Wassarman, P.M.: Eukaryotic chromosome replication: A close-up of the replication fork. Annual Review Biochemistry 49: 627-666 (1980).
Shelton, E.R., Wassarman, P.M., and DePamphilis, M.L.: Structure, spacing and phasing of nucleosomes on isolated forms of mature simian virus 40 chromosomes. Journal Biological Chemistry 255: 771-782 (1980).
Bleil, J.D. and Wassarman, P.M.: Structure and function of the zona pellucida: Identification and characterization of the proteins of the mouse oocyte’s zona pellucida. Developmental Biology 76: 185-202 (1980).
Bleil, J.D. and Wassarman, P.M.: Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proceedings National Academy of Sciences, USA 77: 1029-1033 (1980).
Bleil, J.D. and Wassarman, P.M.: Mammalian sperm-egg interaction: Identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell 20: 873-882 (1980).
Herman, T.M., DePamphilis, M.L., and Wassarman, P.M.: Structure of chromatin at DNA replication forks: Location of the first nucleosomes on newly synthesized Simian virus 40 DNA. Biochemistry 20: 621-630 (1981).
Greve, J.M., Salzmann, G.S., Roller, R.J., and Wassarman, P.M.: Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell 31: 749-759 (1982).
DePamphilis, M.L. and Wassarman, P.M.: The organization and replication of Papovavirus DNA and chromatin. In, The Organization and Replication of Viral DNA, (A.S. Kaplan, ed.), pp. 37-114 (1982).
Salzmann, G.S., Greve, J.M., Roller, R.J., and Wassarman, P.M.: Biosynthesis of the sperm receptor during oogenesis in the mouse. EMBO Journal 2: 1451-1456 (1983).
Cusick, M.E., DePamphilis, M.L., and Wassarman, P.M.: Dispersive segregation of nucleosomes during replication of simian virus 40 chromosomes. Journal Molecular Biology 178: 249-261 (1984).
Greve, J.M. and Wassarman, P.M.: Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. Journal Molecular Biology 181: 253-264 (1985).
Florman, H.M. and Wassarman, P.M.: O-Linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell 41: 313-324 (1985).
Wassarman, P.M., Bleil, J.D., Florman, H.M., Greve, J.M. et al.: The mouse egg’s sperm receptor: What is it and how does it work? In, Molecular Biology of Development, Cold Spring Harbor Symposium Quantitative Biology 50: 11-18 (1985).
Bleil, J.D. and Wassarman, P.M.: Autoradiographic visualization of the mouse egg’s sperm receptor bound to sperm. Journal Cell Biology 102: 1363-1371 (1986).
Wassarman, P.M.: The biology and chemistry of fertilization. Science 235: 553-560 (1987).
Chalifour, L.E., Wirak, D.O., Hansen, U., Wassarman, P.M., and DePamphilis, M.L.: Cis- and trans-acting sequences required for the expression of Simian virus 40 genes in mouse oocytes. Genes and Development 1: 1096-1106 (1987).
Wassarman, P.M.: Early events in mammalian fertilization. Annual Review Cell Biology 3: 109-142 (1987).
Bleil, J.D., Greve, J.M., and Wassarman, P.M.: Identification of a secondary sperm receptor in the mouse egg zona pellucida: Role in maintenance of binding of acrosome-reacted sperm to eggs. Developmental Biology 128: 376-385 (1988).
Kinloch, R.A., Roller, R.J., Fimiani, C.M., Wassarman, D.A., and Wassarman, P.M.: Primary structure of the mouse sperm receptor’s polypeptide chain determined by genomic cloning. Proceedings National Academy of Sciences, USA 85: 6409-6413 (1988).
Wassarman, P.M.: Zona pellucida glycoproteins. Annual Review Biochemistry 57: 415-442 (1988).
Wassarman, P.M.: Fertilization in mammals. Scientific American 255: 78-84 (December, 1988).
Wassarman, P.M. and Kornberg, R.D. (Eds.): Nucleosomes. Methods in Enzymology 170: 683 pp. (1989).
1970 - 1979
Wassarman, P.M. and Lentz, P.J.: The interaction of tetraiodofluorescein with dogfish muscle lactate dehydrogenase: A chemical and X-ray crystallographic investigation. Journal Molecular Biology 60: 509-523 (1971).
Wassarman, P.M. and Burgner, J.W.: Kinetics of unfolding of dogfish muscle lactate dehydrogenase in the presence of guanidine hydrochloride. Journal Molecular Biology 67: 537-542 (1972).
Wassarman, P.M., Hollinger, T.G., and Smith, L.D.: RNA polymerases in the germinal vesicle contents of Rana pipiens oocytes. Nature 240: 208-210 (1972).
Sorensen, R.A. and Wassarman, P.M.: Relationship between growth and meiotic maturation of the mouse oocyte. Developmental Biology 50: 531-536 (1976).
Wassarman, P.M. and Letourneau, G.E.: RNA synthesis in fully grown mouse oocytes. Nature 261: 73-74 (1976).
Wassarman, P.M., Josefowicz, W.J., and Letourneau, G.E.: Meiotic maturation of mouse oocytes in vitro: Inhibition of maturation at specific stages of nuclear progression. Journal Cell Science 22: 531-545 (1976).
Schultz, R.M. and Wassarman, P.M.: Specific changes in the pattern of protein synthesis during meiotic maturation of the mammalian oocyte in vitro. Proceedings National Academy of Sciences, USA 74: 538-542 (1977).
Wassarman, P.M., Ukena, T.E., Josefowicz, W.J., and Karnovsky, M.J.: Asymmetrical distribution of microvilli in cytochalasin B induced pseudo-cleavage of mouse oocytes. Nature 265: 742-744 (1977).
Schultz, R.M., LaMarca, M.J., and Wassarman, P.M.: Absolute rates of protein synthesis during meiotic maturation of mouse oocytes in vitro. Proceedings National Academy of Sciences, USA 75: 4160- 4164 (1978).
Shelton, E.R., Wassarman, P.M., and DePamphilis, M.L.: The structure of Simian virus 40 chromosomes in nuclei from infected monkey cells. Journal Molecular Biology 125: 491-510 (1978).
Team

Paul M. Wassarman, Ph.D., Brandeis University (Biochemistry), is a Full Professor at ISMMS. He was a Helen Hay Whitney Foundation Postdoctoral Fellow at the MRC Laboratory of Molecular Biology, Cambridge, England, and a Rockefeller Foundation Special Research Fellow at Harvard Medical School. Previously a faculty member in the Department of Biological Chemistry at Harvard Medical School and the Department of Cell and Developmental Biology at the Roche Institute of Molecular Biology, he has more than 40 years of research experience working on fertilization and extracellular coat proteins of mammalian and non-mammalian eggs and has published more than 240 primary papers and reviews. He is Series Editor of Current Topics in Developmental Biology published five-times annually by Academic Press/Elsevier, editor of more than a dozen books and periodicals, and author of A Place in History: The Biography of John C. Kendrew published by Oxford University Press in 2020.
E-mail: paul.wassarman@mssm.edu

Eveline S. Litscher, Ph.D., University of Zürich (Cell Biology), is an Assistant Professor at ISMMS. Previously a Swiss National Science Foundation Postdoctoral Fellow in the Department of Cell and Developmental Biology at the Roche Institute of Molecular Biology, she has more than 30 years of research experience working on fertilization and extracellular coat proteins of mammalian and non-mammalian eggs and has published more than 50 primary papers and reviews. She is the author of A Guide to Zona Pellucida Domain Proteins published by John Wiley and Sons in 2015.
E-mail: eveline.litscher@mssm.edu
Alumni
Graduate Students
Jeffrey Bleil, Ph.D., Harvard University
George Salzmann, Ph.D., Harvard University
Stephanie Cascio, Ph.D., Harvard University
Michael Cusick, Ph.D., Harvard University
Richard Roller, Ph.D., Harvard University
Earl Shelton, Ph.D., Harvard University
Huayu Qi, Ph.D., Mount Sinai Medical School
Zev Williams, Ph.D., Mount Sinai Medical Schoool
Postdoctoral/Sabbatical Fellows
Ralph Sorensen, Ph.D., Yale University
Darwin Keichline, Ph.D., University of Tennessee
Richard Schultz, Ph.D., Harvard University
Timothy Herman, Ph.D., Oregon State University
Lois Tack, Ph.D., Johns Hopkins University
Keun-Su Lee, Ph.D., Yale University
Dana Wirak, Ph.D., University of Michigan
Lorraine Chalifour, Ph.D., University of Manitoba
Jeffrey Greve, Ph.D., Washington University
Harvey Florman, Ph.D., University of Pennsylvania
Jeffrey Bleil, Ph.D., Harvard University
Rosario Perona, Ph.D., University of Madrid
Fred Samuels, Ph.D., Albert Einstein University
Ross Kinloch, Ph.D., Glasgow University
Christopher Moller, Ph.D., University of Pennsylvania
Rita Rein, Ph.D., Cologne University
Monica Vazquez, Ph.D., Buenos Aires University
Betina Ruiz-Seiler, Ph.D., Free University of Berlin
Marco Gonzalez-Martinez, Ph.D., University of Mexico City
Sergio Lira, Ph.D., University of California-San Diego
Thomas Rosiere, Ph.D., University of Illinois
Michael Schickler, Ph.D., Weizmann Institute
Andreas Batzer, Ph.D., University of Konstanz
Yutaka Sakai, Ph.D., University of Tokyo
Eveline Litscher, Ph.D., University of Zürich
Chengyu Liu, Ph.D., University of California-Irvine
Marla Weetall, Ph.D., Cornell University
Jie Chen, Ph.D., Tufts University Medical School
Sven Johan Hyllner, Ph.D., University of Goteborg
Theresa Matkovits, Ph.D., New Jersey Medical School
Natalie Cohen, Ph.D., Bar-Ilan University
Luca Del Giacco, Ph.D., University of Milan
Luca Jovine, Ph.D., MRC-LMB, Cambridge University
Costel Darie, Ph.D., University of Freiburg
Michael LaMarca, Ph.D., Professor, Lawrence University
Nathan Sharon, Ph.D., Professor, Weizmann Institute
Ruth Shalgi, Ph.D., Professor, Tel-Aviv University
Research Technicians
Stephen Mortillo, M.S.
Carolyn Fimiani, B.A.
Wendy Josefowicz, B.S.
Gail Letourneau, B.S.
Tracy Pirozzi, M.S.
Eileen Herlihy, B.S.
Pamela Turner, B.S.
Nisha Shah, B.S.
Elain Wu, B.S.
Kari Juntunen, M.S.
Annamari Vuorela, M.S.
Paul M. Wassarman
Department of Cell, Developmental, and Regenerative Biology
Icahn School of Medicine at Mount Sinai
Annenberg 25-30B, Box 1020
1468 Madison Avenue
New York, NY 10029-6574
Office: (212) 241-8616/8620
E-mail: paul.wassarman@mssm.edu
