Developing a Genetically Based Precision Medicine Approach in Psychiatry
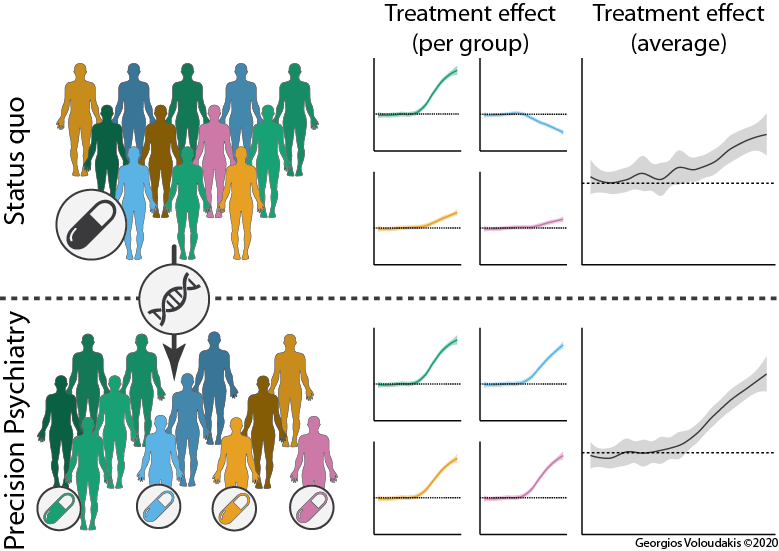
Our group aims to leverage genomics to improve outcomes for patients suffering from neuropsychiatric disorders. Towards building a precision medicine approach for psychiatry, we are constructing genomic predictive models of the human brain to understand how genetic variation affects the transcriptional and epigenetic landscape. Subsequently, we apply these models to:
- Existing genetic studies to understand more about the biology of disease and to improve existing drug development and repurposing approaches
- Biobanks with electronic health record information, such as the Million Veteran Program (MVP), All of Us, BioMe and UK Biobank (UKBB) to identify disease subgroups, stratify risk and model medication response
We collaborate closely with the Roussos, Akbarian, Björkegren, Bigdeli, Fanous, Harvey, Davis and Smoller laboratories towards common milestones in the field.

Georgios Voloudakis, M.D., Ph.D.
Pronouns: he/him
Icahn School of Medicine at Mount Sinai
• Associate Professor, Departments of Psychiatry, Genetics and Genomic Sciences and Artificial Intelligence and Human Health
• Director, Translational Bioinformatics and Precision Therapeutics Group, Center for Disease Neurogenomics
• Member, The Friedman Brain Institute
James J. Peters VA Medical Center
• Investigator, VISN2 Mental Illness Research, Education and Clinical Centers (MIRECC)
• Associate Director, Center for Precision Medicine and Translational Therapeutics (CPM2T)![]()
![]()
Current Projects
Towards achieving our long-term goal of building a framework for genetically based precision and personalized medicine approaches in psychiatry, we are developing and optimizing the necessary tools and strategies for:
- Improved genomic feature imputation
- Expanding imputation approaches beyond the transcriptome to include the epigenome and other multiomics datasets
- data-driven integration of imputed molecular features
- Deep phenotyping of individuals based on information of Electronic Health Records
- Computational drug repurposing and drug development
- Better collaboration between sites
Genetics of neuropsychiatric disorders. Via collaborations with multiple groups we are contributing to the elucidation of the genetic architecture of multiple disorders such as schizophrenia, bipolar disorder, suicidal behavior, etc. across diverse population groups.
Reducing the stigma of mental health disorders by understanding the pleiotropy of their risk variants. Neuropsychiatric disorders are moderately to highly heritable and a lot of genetic variants that confer risk have been identified over the past two decades. Some of these variants are quite common and it would make sense from an evolutionary perspective for them to have “survived” because they also have beneficial functions either for the individual or for the society. We aim to identify these trade-offs to understand the potentially beneficial impact they have in our world and to our societies.
Funding:
NIH, NIA, R01AG078657, Identifying genetically driven gene dysregulation in Alzheimer’s disease and related dementias using statistical data integration
NIH, NIMH, K08MH122911, Characterizing and targeting subphenotypes of schizophrenia and bipolar disorder via individually imputed tissue and cell-type specific transcriptomes
BBRF, Elucidating the genetic basis of suicidal behavior (completed)
NIH, NIA, P50AG005138 (Parent), Pilot 35-3, Identifying genetically-driven microglial gene expression changes in AD (completed)
Leon Levy Foundation, Neuroscience Fellowship (completed)
Recent and Selected Publications
Development of statistical genetics and translational genomics approaches for gene target prioritization and computational drug repurposing
- Voloudakis G✉, Vicari JM, Venkatesh S, Hoffman GE, Dobrindt K, …, Mount Sinai COVID-19 Biobank, VA Million Veteran Program COVID-19 Science Initiative, Brennand KJ, Fullard JF, Roussos P✉. A translational genomics approach identifies IL10RB as the top candidate gene target for COVID-19 susceptibility. NPJ Genomic Medicine. 2022 Sep; 7, 52 (2022).
- Zhang W‡, Voloudakis G‡, Rajagopal VM, Reahead B, Dudley JT, Schadt EE, Bjorkegren JLM, Kim Y, Fullard JF, Hoffman GE, Roussos P. Integrative Transcriptome Imputation Reveals Tissue-Specific and Shared Biological Mechanisms Mediating Susceptibility to Complex Traits. Nat Commun. 2019 Aug 23;10(1):3834.
Leveraging genotyped biobanks with electronic medical records to empower genetic discovery and pleiotropy interrogation for neuropsychiatric traits
- Burstein D, Griffen T, Therrien K, Bendl J, Venkatesh S, Dong P, Modabbernia A, Zeng B, Mathur D, Hoffman G, Sysko R, Hildebrandt T, Voloudakis G‡✉, Roussos P‡✉. Genome-wide analysis of a model-derived binge eating disorder phenotype identifies risk loci and implicates iron metabolism. Nature Genetics. 2023 Sep;55(9):1462-1470.
- Rajagopal VM, Ganna A, Coleman JRI, Allegrini A, Voloudakis G, Grove J, Als TD, …, Breen G, Roussos P, Plomin R, Agerbo E, Børglum AD, Demontis D. Genome-wide association study of school grades identifies genetic overlap between language ability, psychopathology and creativity. Sci Rep. 2023 Jan 9;13(1):429.
- Bigdeli TB‡, Voloudakis G‡, Barr PB, Gorman BR, Genovese G, Peterson RE, Burstein DE, Velicu VI, Li Y, Gupta R, Mattheisen M, Tomasi S, …, Fanous AH, Harvey PD, Roussos P. Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia, Bipolar Disorder, and Depression in the VA Health Care System. JAMA Psychiatry. 2022 Sep 14;79(11):1092-1101
Transcriptome- and epigenome-wide association studies
- Als TD, Kurki MI, Grove J, Voloudakis G, Therrien K, Tasanko E, …, Hovatta I, Roussos P, Daly MJ, Mors O, Palotie A, Børglum AD. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nature Medicine. 2023 Jul;29(7):1832-1844.
- Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT, Farajzadeh L, Voloudakis G, Bendl J, …, Roussos P, Franke B, Werge T, Neale BM, Stefansson K, Børglum AD. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genetics. 2023 Feb;55(2):198-208. doi: 10.1038/s41588-022-01285-8. Epub 2023 Jan 26. PubMed PMID: 36702997.
- Mattheisen M‡, Grove J‡, Als TD‡, Martin J‡, Voloudakis G, Meier S, …, Robinson E, Roussos P, Neale BM, Daly MJ, Børglum AD. Identification of shared and differentiating genetic risk for autism spectrum disorder, attention deficit hyperactivity disorder and case subgroups. Nature Genetics. 2022 Oct;54(10):1470-1478
Molecular Pathophysiology of Neuropsychiatric Disorders
- Yoon YJ‡, Voloudakis G‡, Doran N, Zhang E, Dimovasili C, Chen L, Shao Z, Darmanis S, Tang C, Tang J, Wang VX, Hof PR, Robakis NK, Georgakopoulos A. PS1 FAD mutants decrease ephrinB2-regulated angiogenic functions, ischemia-induced brain neovascularization and neuronal survival. Mol Psychiatry 2020. https://doi.org/10.1038/s41380-020-0812-7
- Warren NA, Voloudakis G, Yoon Y, Robakis NK, Georgakopoulos A. The product of the γ-secretase processing of ephrinB2 regulates VE-cadherin complexes and angiogenesis. Cell Mol Life Sci. 2018 Aug;75(15):2813-2826.
- Huang Q, Voloudakis G, Ren Y, Yoon Y, Zhang E, Kajiwara Y, Shao Z, Xuan Z, Lebedev D, Georgakopoulos A, Robakis NK. Presenilin1/γ-secretase protects neurons from glucose deprivation-induced death by regulating miR-212 and PEA15. FASEB J. 2018 Jan;32(1):243-253.
‡ Authors contributed equally to this work; ✉ Corresponding authors
Team

Sanan Venkatesh, BSc
Graduate student (GDS)
sanan.venkatesh {at} icahn.mssm.edu
07/01/2020 – Present
Scholar | LinkedIn
Co-mentor: Panos Roussos
– 12/2020 Society for Neuroscience (SfN) Trainee Professional Development Award (TPDA)

– 09/2020 Million Veteran Program (MVP) Early Stage Investigator (ESI) Award





chenyu.liu {at} icahn.mssm.edu
03/21/2023 – Present

bukola.ajanaku {at} icahn.mssm.edu
07/06/2023 – Present

joonho.seo {at} mssm.edu
07/14/2023 – Present



marios.anyfantakis {at} mssm.edu
10/02/2023 – Present

neha.ahmed {at} mssm.edu
11/13/2023 – Present

fotios.tsetsos {at} mssm.edu
03/04/2024 – Present

jamie.bennett {at} mssm.edu
03/25/2024 – Present


Want to join our team?
Open positions: none at this moment.
We are looking for talented scientists and engineers. Email: georgios.voloudakis {at} mssm.edu to discuss career opportunities.
- 2021-07-01 to 2023-08-27 (High School Student): Ciella Angel-Lalanne. Scholar | LinkedIn
Hess CSM Floor 9
1470 Madison Ave
Phone:
Office: (212) 659-9282
Lab:
georgios.voloudakis {at} mssm.edu
