Research topics
We are a team of chemists, material scientists, and immunologists aiming to advance innate immunotherapy. We do this by designing novel nanotherapeutics and imaging tracers, and studying these in animal models of organ transplantation, cancer, and cardiovascular disease.
Developing novel PET probes
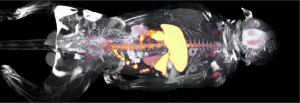 Biomedical imaging enables non-invasively study of (bio)materials in vivo. The high sensitivity and quantitative nature of PET imaging make this an especially powerful approach. We routinely radiolabel nanotherapeutics and biomolecules to evaluate their pharmacokinetics and biodistribution in small and large animal models. We also study the immunological effects of disease and our interventions using novel immuno-PET probes. For instance, we developed nanobody-based imaging protocols for longitudinally tracking distinct immune cell subsets in murine models of cancer, myocardial infarction, and heart transplantation. Lastly, to gain molecular-level insights, we leverage synthetic chemistry to develop small molecule radiotracers for monitoring metabolic and epigenetic processes. Our imaging studies are performed using the state-of-the-art facilities of Mount Sinai’s BioMedical Engineering and Imaging Institute (PET/CT and PET/MRI).
Biomedical imaging enables non-invasively study of (bio)materials in vivo. The high sensitivity and quantitative nature of PET imaging make this an especially powerful approach. We routinely radiolabel nanotherapeutics and biomolecules to evaluate their pharmacokinetics and biodistribution in small and large animal models. We also study the immunological effects of disease and our interventions using novel immuno-PET probes. For instance, we developed nanobody-based imaging protocols for longitudinally tracking distinct immune cell subsets in murine models of cancer, myocardial infarction, and heart transplantation. Lastly, to gain molecular-level insights, we leverage synthetic chemistry to develop small molecule radiotracers for monitoring metabolic and epigenetic processes. Our imaging studies are performed using the state-of-the-art facilities of Mount Sinai’s BioMedical Engineering and Imaging Institute (PET/CT and PET/MRI).
Studying and modulating trained immunity
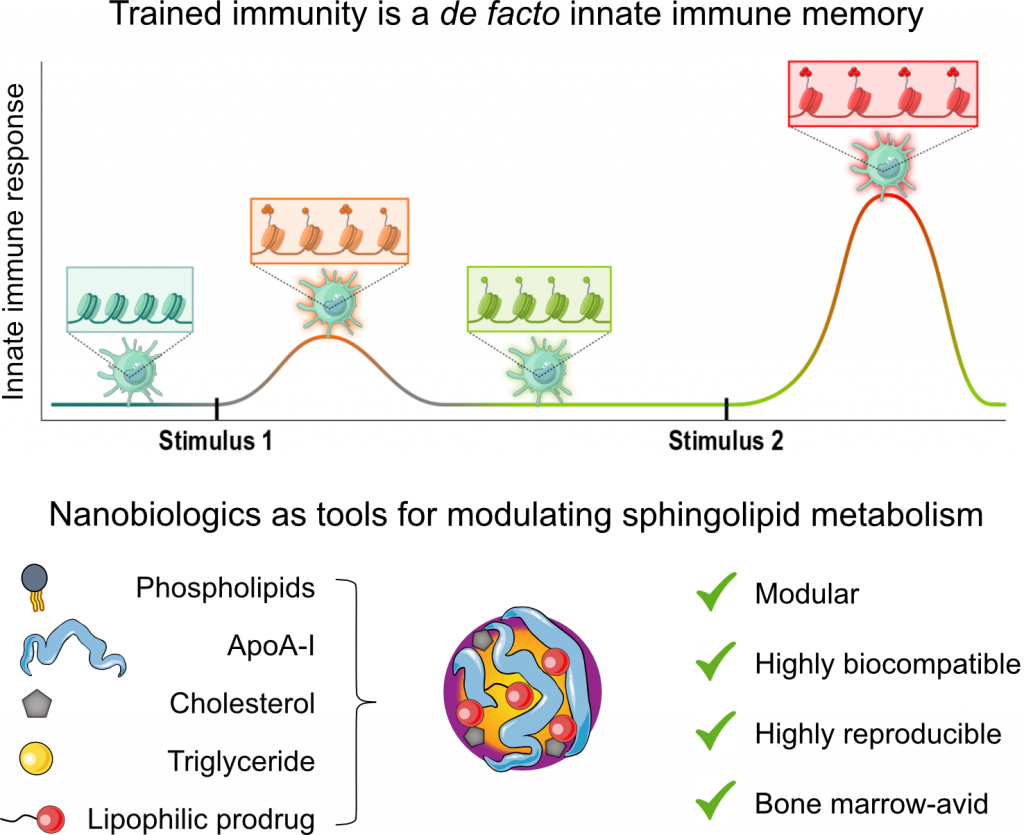 Immunological memory has long been regarded as an exclusive hallmark of the adaptive immune system. However, this dogma has been challenged by a growing body of literature demonstrating an innate immune memory, termed ‘trained immunity. Trained immunity is regulated by epigenetic and metabolic changes in myeloid cells and their progenitors in the bone marrow. These modifications endow innate immune cells with the ability to ‘remember’ prior stimuli (e.g., encounters with a pathogen or DAMP) and hyperrespond to subsequent stimuli, both related and unrelated. Judicious regulation of trained immunity in vivo holds great potential for treating conditions characterized by dysregulated immune systems, including cancer, infectious diseases, and organ transplant rejection. However, this strategy requires delivering trained immunity-regulating drugs to myeloid progenitors in the bone marrow. To achieve this, we have designed lipoprotein-based nanocarriers, termed ‘nanobiologics’. Nanobiologics have a high myeloid cell-avidity and can be loaded with diverse small molecule drugs using a prodrug strategy developed by us. We have extensively used nanobiologics to study and treat a broad range of diseases in mouse models, larger animals, and on human cells. We also actively work on improving the targeting of RNA-loaded lipid nanoparticles.
Immunological memory has long been regarded as an exclusive hallmark of the adaptive immune system. However, this dogma has been challenged by a growing body of literature demonstrating an innate immune memory, termed ‘trained immunity. Trained immunity is regulated by epigenetic and metabolic changes in myeloid cells and their progenitors in the bone marrow. These modifications endow innate immune cells with the ability to ‘remember’ prior stimuli (e.g., encounters with a pathogen or DAMP) and hyperrespond to subsequent stimuli, both related and unrelated. Judicious regulation of trained immunity in vivo holds great potential for treating conditions characterized by dysregulated immune systems, including cancer, infectious diseases, and organ transplant rejection. However, this strategy requires delivering trained immunity-regulating drugs to myeloid progenitors in the bone marrow. To achieve this, we have designed lipoprotein-based nanocarriers, termed ‘nanobiologics’. Nanobiologics have a high myeloid cell-avidity and can be loaded with diverse small molecule drugs using a prodrug strategy developed by us. We have extensively used nanobiologics to study and treat a broad range of diseases in mouse models, larger animals, and on human cells. We also actively work on improving the targeting of RNA-loaded lipid nanoparticles.
