Interventional Clinical Trial Design, Analysis, and Conduct [Free of Charge]
Trial Design
- Formulate testable hypotheses
- Assist with selecting appropriate endpoints
- Propose innovative study design best suited to test primary objective and endpoint
- Compute sample size to appropriately power the study to detect clinically meaningful effect for primary endpoint.
- Develop Treatment allocation and randomization plan(simple, permuted blocks, minimization, dynamic, adaptive, etc.)
- Compute stopping rules for interim assessments of futility, efficacy and safety
- Liaise with regulatory authorities (FDA/EMA), industry sponsors, and institutional committees to ensure appropriate statistical development.
Interventional Clinical Trial Protocol Development
- Develop and write a statistical analysis plan (SAP) by identifying appropriate and novel statistical methods to analyze each endpoint.
- Write all statistical sections for protocols and collaborate on protocol development before submission to the Disease Focus Group (DFG), PRMC and DSMC.
To develop an interventional clinical trial protocol submit request for ‘protocol development’
A statistician will be assigned based on the following DFG designation
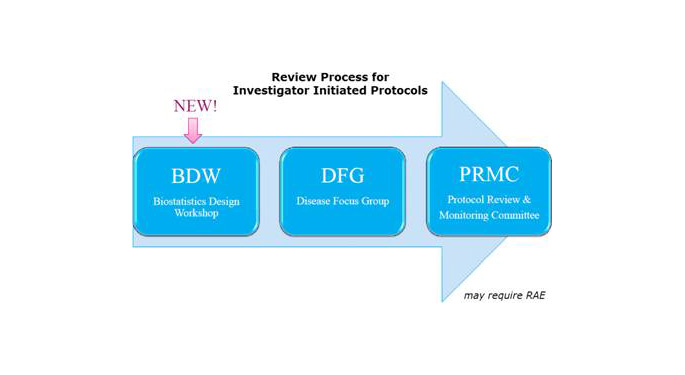
Following are itemized steps of the BDW review process:
1. All Interventional Investigator initiated research protocols must be reviewed by the Biostatistics Design Workshop (BDW) prior to submission to the DFG with exception for protocols that underwent scientific and biostatistical review by one of the following organizations.
2. Collaborators should submit requests for statistical support for ‘PROTOCOL DEVELOPMENT’ through service request form
3. DFG biostatistician is assigned and develops statistical sections of protocol
a. Only Interventional (I-IIT) require BDW (DFG and PRMC) approval and need to contain the Essential Elements of a Protocol (industry provided protocol template), or be in proper PRMC format: Protocol Template
b. The IRB format (HRP-503a) is NOT an acceptable format for I-IITs
4. DFG Biostatistician submits protocol for BDW review when it is deemed ready by biostatistician and PI
5. Once a protocol is approved by BDW, it can be presented at DFG
a. On BDW approval, all track changes are accepted
b. A version number and date of BDW approval is added to the protocol
c. The BDW approved protocol is emailed to the PI, DFG Chairman and PRMC administrators
6. After DFG approval, the protocol can be submitted for PRMC review (contact Venesha White Venesha.white@mssm.edu for information on this submission process)
Trial Monitoring
- Developing statistical programs for data monitoring
- Conduct Interim analyses for futility, efficacy, and safety as specified in the protocol and make recommendations for decision making about stopping or continuing the trial.
- Review of electronic data capture forms developed by CTO in RedCAP
Trial Conduct and Analysis
- Perform analysis outlined in SAP using state of the art software tools.
- Generate tables, figures and summaries of results for publication or presentation.
- Co-author manuscripts including drafting a statistical methods section and critically revising the entire manuscript with special consideration on interpretation of statistical results.
- Respond to reviewers’ comments on coauthored manuscripts.
Clinical Trial Review and Safety Monitoring [Free of Charge]
Clinical Trial Review and Safety Monitoring
- Biostatistics Shared Resource members serve on the TCI Protocol Review and Monitoring Committee (PRMC) to provide critical input into proposed and ongoing institutional investigator initiated and external industry sponsored clinical trials.
- Biostatistics Shared Resource members serve on the TCI Data Safety and Monitoring Committee (DSMC) to review study level and individual level data related to safety, quality and timeliness of data entry, patient recruitment, accrual and retention, statistical outcomes, unanticipated problems and protocol violations, serious adverse events and overall conduct of the trial. – link to DSMC website materials.
Training & Education [Free of Charge]
Training & Education
- Our experienced faculty and staff provide the following mentoring and training to graduate students, residents, fellows, faculty and staff:
- Design and analytic methods immersive training through workshops and seminars (IHDS Seminar Series, BDAW, BSR Walk-in Clinic, BSR Journal Club)
- Mentoring residents, fellows and junior investigators on young investigator awards and early career development training grants
- Lectures covering variety of topics, including but not limited to: statistical methods in medical literature, power and sample size calculations, database design, clinical trials design, test development and evaluation, and statistical computing.
Grant Proposal Development [Grant Budget]
Grant Proposal Development
To encourage and facilitate more research activities the Biostatistics Shared Resource will provide research design and data analysis plan support and expertise in the development of extramural grant proposals. Excluding extensive preliminary data analysis, the support is currently free of charge as long as an adequate percentage FTE of the participating faculty and staff is built into the grant budget.
** Development and Write-Up is free of Charge with support provided by the Cancer Center Support Grant as long as an adequate % FTE of the participating faculty and staff is built into the grant budget.
Observational Research Study Design, Analysis, and Dissemination [Fee for Service, LTCC]
Observational Research Study Design, Analysis, and Dissemination
To encourage and facilitate more research activities the Biostatistics Shared Resource will provide research design and data analysis plan support and expertise in the development of extramural grant proposals. Excluding extensive preliminary data analysis, the support is currently free of charge as long as an adequate percentage FTE of the participating faculty and staff is built into the grant budget.
Methods Development and Algorithm Refinement [Fee for Service, LTCC]
Methods Development and Algorithm Refinement
Tailored statistical tool development to address specific research questions and challenges
Algorithm refinement to make opensource algorithms work for Microscopy and Advanced Imaging Core users
Data Source Identification and Acquisition
Data Source Identification and Acquisition
- Housing and maintaining a repository of administrative data and information about the data sources
- Training investigators on the ethical and secure use of administrative data
- Providing leadership on study design and analysis of administrative data
- Facilitating collaboration among interdisciplinary investigators
- Advancing the effective use of data to inform researchers, policymakers and the public about health and healthcare
Find a description of the financial models for TCI-BSR services here:
