Policies
TCI-BSR has the following polices to guide effective and equitable collaboration:
Policies
Public Acknowledgement of Cancer Center Support Grant
All publications, press releases and/or presentations that include results, services or products generated by the TCI-BSR must include the following acknowledgement in adherence with NIH policy:
“Services and products in support of this research project were generated by the Tisch Cancer Institute Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA0196521.”
In addition, publications that acknowledge the CCSG must also comply with the NIH Public Access Policy which states that a PubMed Central ID (PMCID) is required when you publish research that was supported by any NIH funds.
Authorship
The mechanism used to provide support does not alter the collaborative atmosphere that the TCI-BSR has fostered over the years. The contribution of each person needs to be evaluated as a manuscript is prepared. Consideration for authorship should be based on the accepted criteria for most medical journals. These criteria generally cite both study design and statistical analysis as intellectual input sufficient for authorship. It is impossible to define every situation in advance; however, it should be clear that reimbursement for time does not preclude or replace authorship.
Collaborating statisticians qualify for authorship on manuscripts and abstracts if the criteria listed below are met. These criteria are derived from the International Council of Medical Journal Editors Requirements, from Criteria for Authorship For Statisticians in Medical Papers, by Parker and Bergman, Stat. Med. 17, 2289–2299 (1998), and from Rutgers. Based on these references, examples of these criteria include, but are not limited to, the following:
- The statistician(s) contributes substantially to the conception and design of the research project (e.g., developing the analysis plan is a substantial contribution to the study design).
- The statistician(s) interprets results and performs statistical analysis, data management, and data analysis.
- The statistician(s) writes part of the manuscript describing which statistical methods were used or assists in writing other sections of the manuscript—including revisions.
Our policy is that MS and PhD statisticians should NOT be listed under acknowledgements if any of the above criteria have been met but should instead be appropriately recognized as authors.
Authorship Order
It is recommended to discuss the authorship order at the initiation of the collaboration. The authorship order of the statisticians depends, as accurately as possible, upon their specific contributions. It is customary to list the faculty statistician as the second author if they have co-led the overall clinical study design, analysis, conduct, and manuscript preparation. If the clinical trial is led by another institution and the BSR faculty is not the lead statistician in the originating institution, then the BSR faculty should be listed as a middle author as long as they have contributed materially to the clinical study design, analysis, conduct, and manuscript preparation.
Payment and Authorship
Payment will not be accepted in lieu of authorship. Authorship is determined by intellectual and scientific contributions, as described earlier. Payment allows the collaborating statistician(s) to engage a project in accordance with NIH guidelines for effort reporting.
Data Management
The TCI-BSR is not responsible for data management.
Here are some guidelines for data management:
Guidelines for Submitting Data to Statisticians
Clinical data management is the process of collection, cleaning, and management of data in compliance with regulatory standards. It is an important part of any research study. Every interventional (I-IIT) should have a clinical data management plan and support of a research study assistant. Statisticians are available to assist in the development of the data management plan by offering guidance on: selecting the right electronic data capture system, the best data collection methods, data entry practices that ensure consistency with paper CRFs, data validation methods and proper transfer of data for statistical analysis. However, biostatisticians are not taking responsibility for data management.
Project Turnaround Timeline
All requests received through our service request form will be assigned to a TCI-BSR statistician within two business days. Once assigned, a statistician will respond to your inquiry within three business days to arrange for the first meeting. Timeline for the work to be done will be discussed in this meeting. For interventional investigator initiated protocols, movement to PRMC requires approval from the ‘Biostatistics Design Workshop’ (BDW), a committee of all statisticians in the TCI-BSR that meets weekly on Thursdays from 2-3 pm. For grant applications, time for development of the statistical sections for a grant also ranges between 2-4 weeks depending on the readiness of the proposal in terms of having all information needed for writing the statistical sections.
https://i2db.wustl.edu/consultation-services/biostatistics-consulting/
Please communicate any deadlines as far in advance as possible. We strive to accommodate deadlines, but it generally takes weeks to months to conduct most analyses or to provide thorough grant preparation assistance. Contact the consulting service as early as possible with an awareness of the following guidelines:
Please schedule a meeting with a consultant as early as possible in the research process. Most requests require at least two months’ notice prior to the requested final deadline.
| Type of Assistance | Minimum Turnaround | |
| Analysis | Typical analyses | 2 months |
| Manuscripts | 2-4 months | |
| Abstracts | 1 month | |
| Grant Applications | P01, SPORE, SCORE grants | 3-6 months |
| Most grants (R01, K awards) | 2-3 months | |
| Smaller statistical sections for grants | 1 month | |
On a case-by-case basis, the Cancer Biostatistics Shared Resource may decline requests that arrive too close to the deadline for us to provide anything useful. Please also consider that more complex designs or analyses will require more time.
General recommendations include:
- Turnaround times for data analyses are contingent upon the data quality, format, amount, complexity, as well as the number and maturity of the study questions addressed.
- Ideally, please contact us several months prior to a grant deadline. If it is a first submission and we do not have adequate time, our contribution will demonstrate statistical involvement but could be incomplete. If it is a re-submission, we need adequate time to improve the score.
- As the deadline approaches, our ability to provide substantial support diminishes.
- Investigators are expected to work with us to help meet their deadlines. Timelines set for providing data, protocol drafts, other information needed by the consultant should be adhered to, or the timeline will be revised.
- Projects run most smoothly when there are frequent, clear and focused communications with a strong commitment from all persons involved.
Initial Consultation Meeting
The initial consultation is intended to enable the consultant to assess the project’s needs and to provide a basic level of consultation to facilitate extensive ongoing support, if necessary. As consultant expertise varies, if a project requires particular skills, we will try to find an appropriate person to fulfill that need. Initial consultation meetings do not typically include computations or analyses.
At the initial consultation meeting, the investigator and consultant(s) should work together to:
- Provide a description of the project.
- Define particular skills required.
- Identify the materials needed by the consultant to perform the work (see guidelines below for materials needed for each type of assistance).
- Identify the hourly billing rate for the project.
- Estimated the investigator’s budget for the project, even if a best guess. This estimate will be used to determine a communication plan about the budget and effort used.
- Estimate timelines, deadline(s), and a communication plan for work needed and workflow (e.g., agendas, flow charts, checklists, shared meeting notes).
- Establish expectations of the work to be performed and deliverables (e.g., analysis section write-up, publication ready tables and figures).
- Discuss expectations of authorship.
Mentor(s) are strongly encouraged to attend the initial consultation meeting for a project that involves their trainee.
Grant Budget Guidelines
The following table provides guidance regarding the percent effort and the level of funding to allocate for biostatisticians on research grants. It is based on the collective experience of faculty and staff biostatisticians in the TCI-BSR and NCI designated Cancer Center shared resource organizations nationwide. These guidelines should serve as a starting point for budget discussions. We strongly recommend that biostatisticians be actively involved throughout the grant proposal development process, including participation in the specification of research objectives and approach; proposal writing; and the making of budgetary decisions for biostatistician and programmer effort commitments, computer and software purchases, and scientific travel.
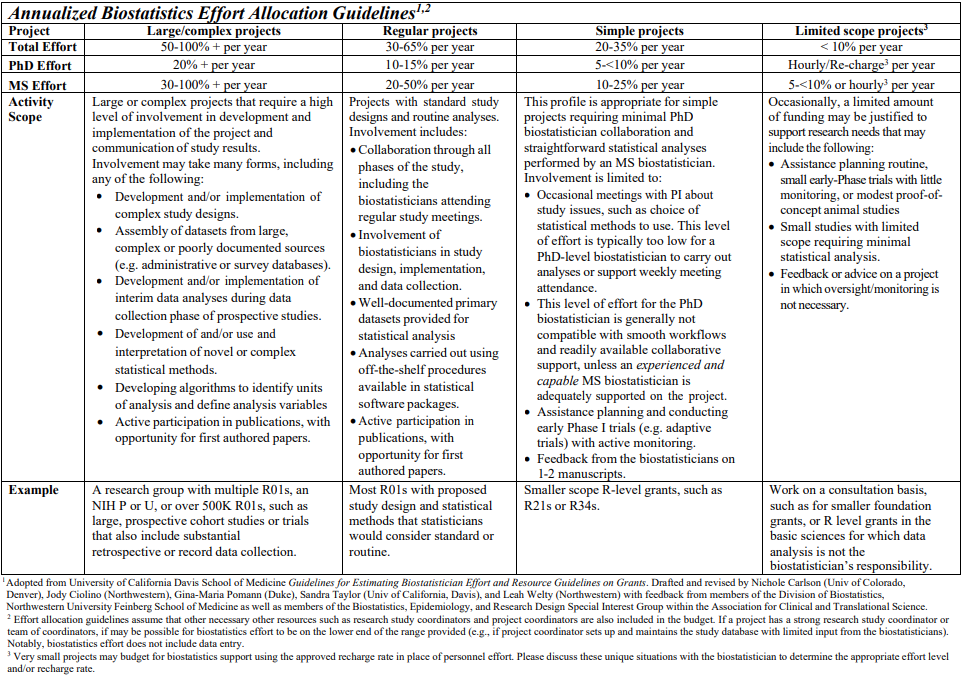
In general, funding for faculty and staff should not fall below 10% of total effort per statistician per time period on a single project. Although there occasionally are valid reasons for a lower level of effort on particular projects, intervals with funded effort falling below 10% require approval by the BSR director.
Grant Funding Cuts
Any changes in percent support made during proposal writing or after research has been funded should be made jointly between the PI and co-investigators/statisticians. If the funding effort falls below 5% in any year of the grant, both the TCI and TCI-BSR directors will be informed.
Data Policy
https://i2db.wustl.edu/consultation-services/biostatistics-consulting/
Data provided to the consultant(s) should adhere to the following guidelines:
- Data provided should be complete and in a format that requires minimal pre-processing and quality control (i.e., ‘cleaning’). All time needed to process the data for analysis will be charged to the investigator. We recommend that the investigator and consultant work together to define the level of data quality control needed.
- To facilitate interpretation of the data, consider providing the consultant(s) with metadata and/or a data dictionary that may include the research instruments, a description of how and when each variable was collected, the data format, history of changes to variables, algorithms for derived data elements and the structure of the database and relationship between elements.
- Protected Health Information (PHI)
- The investigator and the consultant(s) should work together to define the level of PHI that is needed for the analyses.
- Consultant(s) must be included as research team members on the Institutional Review Board (IRB) project for studies where they are provided data that includes PHI.
- Unless necessary for analysis, data should not contain PHI such as names, addresses, medical record numbers, and social security numbers. It is incumbent upon the researcher to remove PHI that is not needed before it is provided to the consultant. Note that ‘hiding’ columns in Excel or other databases does not constitute removal.
Prioritization Policy
The TCI-BSR is available to any cancer investigator in the Icahn School of Medicine at Mount Sinai, whether you are a basic scientist, clinician, epidemiologist, psychologist, behavioral scientist, or other type of researcher. As an investigator you are able to access the facility via online requests for new projects through the facility website or by contacting the members directly for work on ongoing investigations.
The shared resource director will respond to online requests for statistical supports using the priority formulation of:
- Planning of Investigator-Initiated clinical studies
- Preparation of NCI/NIH grant submission
- Analysis of NCI/NIH funded projects (presentation to scientific meetings and manuscript submission are given higher prioritization)
- Preparation of grant submission to non-NIH agencies
- Analysis for projects funded by non-NIH agencies (presentation to scientific meetings and manuscript submission are given higher prioritization
- All others (unfunded exploratory or training related work)
E-mail
Erin.Moshier@mountsinai.org
Request Services
Submit Request
Address
Icahn School of Medicine at Mount Sinai
1425 Madison Avenue, 2nd Floor, Room L2-70
New York, NY 10029
