Purpose: The BDW is comprised of statisticians from the TCI Biostatistics Shared Resource and provides a forum for statistical collaboration on the development, design, and analysis of interventional investigator initiated (I-IIT) protocols. It is the first step in the I-IIT process flow and BDW approval is required before submission of protocols to DFG meetings.
Eligibility for BDW Review
A protocol requires BDW review only if it meets both of the following criteria:
- Interventional Study
A prospective study in which individuals are assigned by an investigator, according to a protocol, to receive specific interventions (e.g., diagnostic, therapeutic, behavioral). Assignment of the intervention may or may not be randomized. Participants are followed, and biomedical and/or health outcomes are assessed.
Note: This includes molecular and imaging diagnostic trials in which the test results affect medical decision-making.
- Investigator-Initiated Study
The Mount Sinai PI must hold the IND or IDE. If the IND or IDE is held by an external sponsor (e.g., pharmaceutical company, cooperative group, or another institution), the protocol is not considered an I-IIT and does not require BDW review.
Exemptions
Protocols that meet the two criteria above (interventional + Sinai-held IND/IDE) do not require BDW review if they have already undergone full scientific and biostatistical review by an approved external organization.
👉 View List of exempting organizations
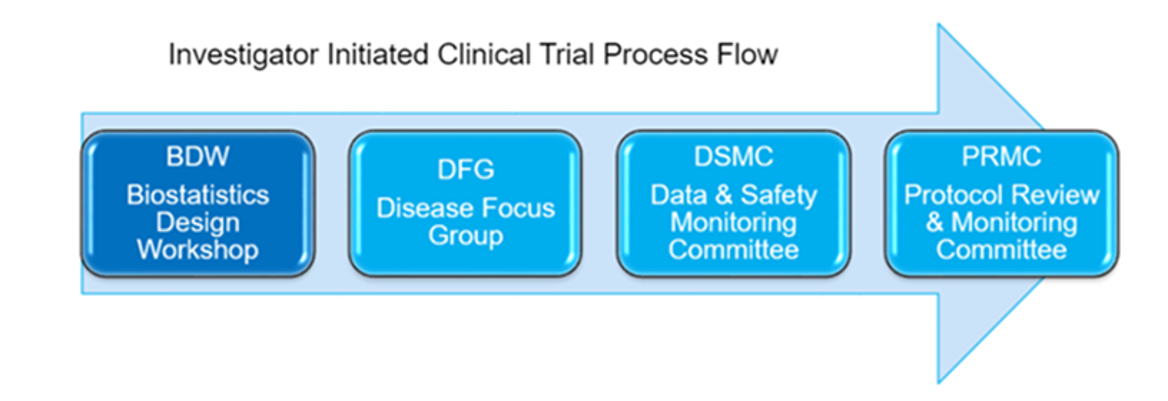
BDW Protocol Development
Early Contact: All investigators are encouraged to contact a biostatistician early in the development stage of their protocol to obtain advice on:
- Study Design: Guidance on structuring the study to ensure it can effectively address the research questions.
- Appropriate Statistical Methods: Recommendations on the best statistical approaches to analyze study endpoints.
- Objective and Endpoint Refinement: Assistance in defining and refining the study’s objectives and endpoints to ensure clarity and measurability.
- Stopping Rules for Futility, Efficacy, and Safety: Development of criteria to determine when a study should be stopped for reasons related to lack of effectiveness, demonstrated benefit, or safety concerns.
- Sample Size: Calculation and justification of the sample size needed to achieve research objectives.
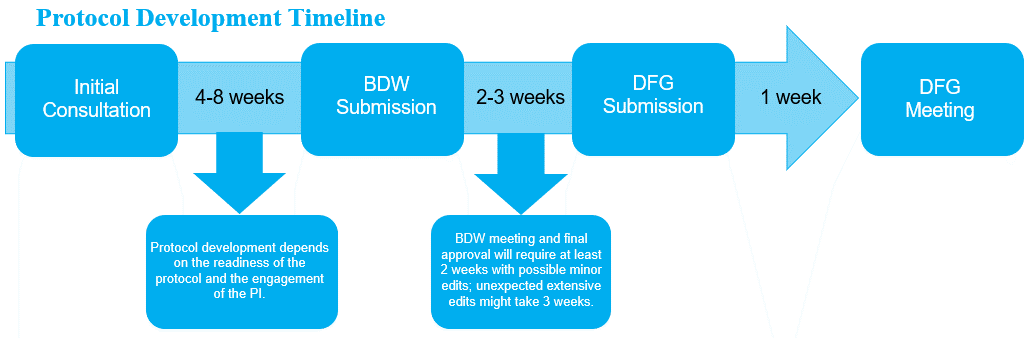
Checklist – Initial Consultation with Biostatistician
- Essentials:
- Primary Objective(s);
- Primary Endpoint(s);
- Previous literature (if available) for the primary endpoint;
- Range of possible sample size;
- Additional Information:
- Secondary and exploratory objectives;
- Secondary end exploratory endpoints;
- Inclusion and Exclusion criteria;
- Schedule of Assessments Table;
- Patient Replacement rules;
- Possible stopping rules for toxicity.
BDW Review
BDW Review:
- The statistician assigned to your protocol will submit and present it for BDW review after completing the statistical sections.
- Investigators do not need to submit or present to the BDW themselves.
- BDW meets weekly on Thursdays from 11:00 am to 12:00 pm.
- Protocols should be submitted by Monday at 5pm of the targeted week for review. If more than two protocols are submitted in a given week, additional protocols will be reviewed the following week.
- BDW feedback will be shared with study statistician by Friday of the same week.
- In case of conditional approval, the protocol with tracked changes and responses addressing issues raised by BDW review should be submitted by the study statistician.
- The protocol does not need to be presented again. Reviewers will accept or raise concerns by email.
- This process takes at least 2 weeks, and it should be initiated at least 4 weeks prior to DFG meeting.
Communication and Approval:
- Statisticians will communicate progress to you throughout the process.
- A final approved version of the protocol will be provided in an email from the BDW coordinator once it is approved and sent to all stakeholders.
Following are itemized steps of the BDW review process:
- All Interventional Investigator initiated research protocols must be reviewed by the Biostatistics Design Workshop (BDW) prior to submission to the DFG with exception for protocols that underwent scientific and biostatistical review by one of the following organizations.
- Collaborators should submit requests for statistical support for ‘PROTOCOL DEVELOPMENT’ through service request form
- DFG biostatistician is assigned and develops statistical sections of protocol
- Only Interventional (I-IIT) require BDW (DFG and PRMC) approval and need to contain the Essential Elements of a Protocol (industry provided protocol template), or be in proper PRMC format: Protocol Template
- The IRB format (HRP-503a) is NOT an acceptable format for I-IITs
- DFG Biostatistician submits protocol for BDW review when it is deemed ready by biostatistician and PI
- Once a protocol is approved by BDW, it can be presented at DFG
- On BDW approval, all track changes are accepted
- A version number and date of BDW approval is added to the protocol
- The BDW approved protocol is emailed to the PI, DFG Chairman and PRMC administrators
- After DFG approval, the protocol can be submitted for PRMC review (contact PRMC@mssm.edu for information on this submission process)
Find a description of the financial models for TCI-BSR services here:
