RASopathies
The RASopathies are a collection of inherited disorders that are characterized by mutations that elevate activity of the RAS/ERK signaling network. Patients present with a broad palette of developmental defects as well as those that emerge postnatally. In addition to reducing quality of life, most patients with these traits encounter some RASopathy-associated morbidities such as hypertrophic cardiomyopathy (HCM), which can be life-threatening. Currently, only symptomatic care is available for affected individuals. Since the biology of the RAS signal transduction pathway is well understood, there is a significant opportunity for developing mechanistic therapies for the RASopathies. For example, several anti-cancer drugs that reduce RAS/ERK pathway activity have been assessed in clinical trials with additional trials ongoing, but to date none has been FDA approved for RASopathies.
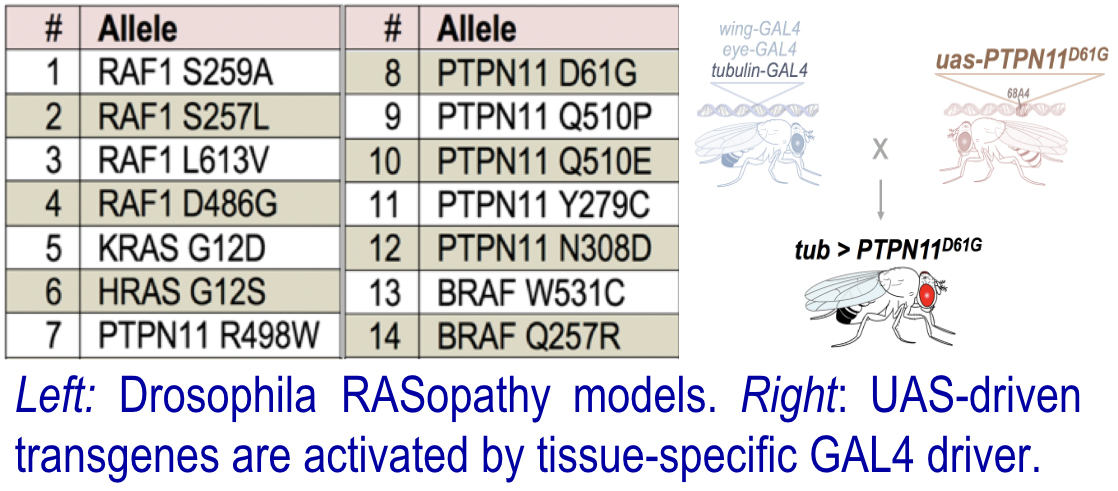
Fly RASopathy Models
To address this continuing unmet need, the Gelb and Cagan laboratories have been working together to identify new lead therapeutics through a novel approach: using a Drosophila RASopathy model as a whole animal screening platform. Screening a large chemical library, we identified a set of four especially promising novel hits, each sitting in novel chemical space. In addition, we have screened a library of FDA-approved drugs and also identified multiple hits.
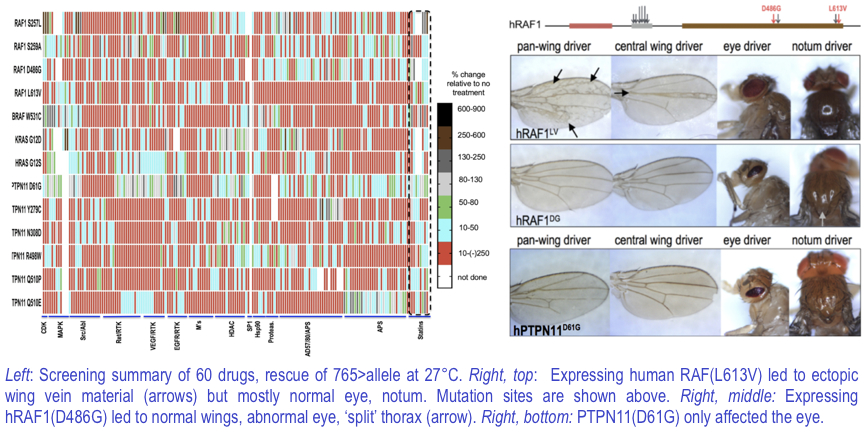
Surprisingly, different RASopathy isoforms responded to different drugs, suggesting the potential for extensive heterogeneity amongst patients. Consistent with this view, different mutations exhibited different effects, including differential effects on eye vs. wing, as shown in the Figure. Again, this points to heterogeneity amongst patients, a key point to consider as we move forward
Relevant References
Assessing the gene-disease association of 19 genes with the RASopathies using the ClinGen gene curation framework. Grant AR, Cushman BJ, Cavé H, Dillon MW, Gelb BD, Gripp KW, Lee JA, Mason-Suares H, Rauen KA, Tartaglia M, Vincent LM, Zenker M. Hum Mutat. 2018 Nov;39(11):1485-1493. doi: 10.1002/humu.23624.
Clinical Presentation and Natural History of Hypertrophic Cardiomyopathy in RASopathies. Calcagni G, Adorisio R, Martinelli S, Grutter G, Baban A, Versacci P, Digilio MC, Drago F, Gelb BD, Tartaglia M, Marino B. Heart Fail Clin. 2018 Apr;14(2):225-235. doi: 10.1016/j.hfc.2017.12.005. Review.
ClinGen’s RASopathy Expert Panel consensus methods for variant interpretation. Gelb BD, Cavé H, Dillon MW, Gripp KW, Lee JA, Mason-Suares H, Rauen KA, Williams B, Zenker M, Vincent LM. Genet Med. 2018 Mar 1. doi: 10.1038/gim.2018.3.
SHOC2 subcellular shuttling requires the KEKE motif-rich region and N-terminal leucine-rich repeat domain and impacts on ERK signalling. Motta M, Chillemi G, Fodale V, Cecchetti S, Coppola S, Stipo S, Cordeddu V, Macioce P, Gelb BD, Tartaglia M. Hum Mol Genet. 2016 Sep 1;25(17):3824-3835. doi: 10.1093/hmg/ddw229. Epub 2016 Jul 27.
The Fourth International Symposium on Genetic Disorders of the Ras/MAPK pathway. Stevenson DA, Schill L, Schoyer L, Andresen BS, Bakker A, Bayrak-Toydemir P, Burkitt-Wright E, Chatfield K, Elefteriou F, Elgersma Y, Fisher MJ, Franz D, Gelb BD, Goriely A, Gripp KW, Hardan AY, Keppler-Noreuil KM, Kerr B, Korf B, Leoni C, McCormick F, Plotkin SR, Rauen KA, Reilly K, Roberts A, Sandler A, Siegel D, Walsh K, Widemann BC. Am J Med Genet A. 2016 Aug;170(8):1959-66. doi: 10.1002/ajmg.a.37723. Epub 2016 May 7.
