Are you interested in participating in one of our human milk studies? Please reach out at: HumanMilkLab@mssm.edu
1) Assessment of the human milk immune response to the maternal RSV vaccine (ABRYSVO)
(we are actively recruiting those who did and did not receive this vaccine)
Pfizer’s RSVpreF vaccine (ABRYSVO), was recently approved for the prevention of serious and severe RSV infection in infants from birth through age 6 months, via maternal immunization (during pregnancy). Maternal immunization provides protection to the infant primarily via transplacentally-transferred maternal antibodies. Additional immunologic benefits may be conferred to human milk-fed infants via maternal antibodies present in the milk of vaccinated individuals, however, human milk specimens from lactating individuals were not collected during any of Pfizer’s clinical studies of ABRYSVO. Our lab has received a Pfizer-sponsored research grant to study vaccine-induced anti-RSV antibodies in milk from lactating, vaccinated individuals over time.
2) Assessment of SARS-CoV-2-reactive antibodies in human milk
Just as New York City was shutting down in early April 2020, I put out a call on social media for study participants, which captured immense media and scientific attention. This attention allowed for a very rapid enrollment of participants into this study (currently there are over 1500 enrolled), and as many participants are local, I was able to collect pilot study samples during the shutdown and quickly optimize a SARS-CoV-2 Spike ELISA . This pilot work determined there was a robust secretory IgA response in all milk samples tested, forming the basis for ultimate NIH-supported work, aimed to determine the titer and durability of the human milk immune response to SARS-CoV-2 infection over time, as well as the functionality of these antibodies, including neutralization capacity and ability to elicit ADCP and mediate the alternative pathway of complement. Beyond the importance of this research to public health, I am conducting pre-clinical work to determine if secretory IgA from milk can act as a therapeutic, using COVID-19 as a model mucosal infection.
As well, I am currently studying the milk immune response to the various COVID-19 vaccines, comparing platforms in order to determine the ideal vaccine for the lactating population globally, as well as characterizing the impact of maternal COVID-19 vaccination on the lipid, protein, and carbohydrate composition of human milk.
3) Fc-mediated antibody function against HIV by relevant primary cell
RV144, the only clinical HIV vaccine trial to show significant efficacy, found that reduced infection rates correlated with non-neutralizing antibodies specific for the V2 region of the HIV Envelope, and that Fc-mediated activities including antibody-dependent cellular phagocytosis (ADCP) correlated with the level of total serum V2 antibodies. I therefore am further exploring the ADCP function of HIV-specific antibodies, and have found that human primary neutrophils exhibit a unique ADCP profile compared to more commonly-assayed but likely less relevant monocyte cell lines. Importantly, neutrophils have been highly understudied in the context of HIV transmission, particularly in the context of mother-to-child transmission (MTCT). Greater than 100,000 MTCTs of HIV via breastfeeding occur annually. However, even in the absence of antiretrovirals, only ~10-15% of infants breastfed by HIV-infected mothers become infected, suggesting a strong protective effect of the milk itself. Unless access to clean water and appropriate infant formula is reliable, the WHO does not recommend cessation of breastfeeding for HIV-infected mothers. Importantly, HIV-specific antibodies in milk have been correlated with reduced MTCT and/or infant death from HIV infection. What remains largely unclear is the contribution of the cellular fraction of human milk to its antiviral qualities. To fill a critical knowledge gap, I therefore aimed to investigate ADCP by human milk leukocytes, hypothesizing this innate immune function may play a key role in minimizing MTCT via breastfeeding. My initial studies found significant ADCP activity in milk that was mainly neutrophil-driven, again highlighting the importance of this understudied cell type. This work formed the basis of NIH–funded work- the comprehensive study of ADCP of HIV by milk leukocytes, examining the impact of target size, antibody class, and stage of lactation., as well as work supported by the Campbell Foundation to perform deep sequencing analysis on milk leukocytes.
4) Maternal vaccination aimed to specifically enhance the human milk immune response
 One overarching goal of our research program is to ultimately design vaccines with protection of infants and young children through breastfeeding in mind. This has been enormously overlooked across the entire field of vaccine design. Our work analyzing the milk Ab response to SARS-CoV-2 found that after infection, robust sIgA is elicited in milk which is exceptionally long-lasting throughout the period of lactation. In contrast, after COVID-19 vaccination, the response is IgG-dominant with moderate IgA and low or undetectable sIgA, all of which wanes quickly within 3-6 months. Furthermore, we have also shown that influenza-specific milk sIgA is poorly boosted after seasonal vaccination. Clearly, intramuscular (IM) vaccines do not elicit significant milk sIgA. Notably, live-attenuated intranasal (IN) influenza vaccine is not better than IM vaccine for eliciting milk sIgA or IgG. There is clearly a true need to design vaccines aimed to elicit a potent, specific sIgA response in milk that will protect human milk-fed infants.
One overarching goal of our research program is to ultimately design vaccines with protection of infants and young children through breastfeeding in mind. This has been enormously overlooked across the entire field of vaccine design. Our work analyzing the milk Ab response to SARS-CoV-2 found that after infection, robust sIgA is elicited in milk which is exceptionally long-lasting throughout the period of lactation. In contrast, after COVID-19 vaccination, the response is IgG-dominant with moderate IgA and low or undetectable sIgA, all of which wanes quickly within 3-6 months. Furthermore, we have also shown that influenza-specific milk sIgA is poorly boosted after seasonal vaccination. Clearly, intramuscular (IM) vaccines do not elicit significant milk sIgA. Notably, live-attenuated intranasal (IN) influenza vaccine is not better than IM vaccine for eliciting milk sIgA or IgG. There is clearly a true need to design vaccines aimed to elicit a potent, specific sIgA response in milk that will protect human milk-fed infants.
In the HIV context, this research is particularly important and unique, as it is evident that although human milk is a vehicle for HIV transmission, its virus-blocking properties are significant, and there may be a role for therapeutic vaccination of HIV-infected mothers to entirely prevent MTCT via breastfeeding. As such, an initial aim of this aspect of my research program is to investigate how best to enhance the potency and function of HIV-specific antibodies in the milk of HIV-infected women, and design HIV vaccines for all women that elicit a robust milk immune response.
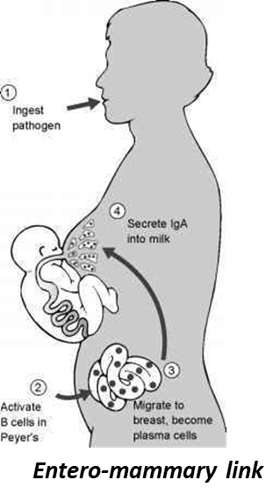
Though human milk antibody is ~90% IgA, nearly all in secretory (s) form (sIgA), most studies have found all HIV-specific antibodies in the milk of HIV-infected women is IgG, a finding which extends to non-human primate (NHP) experiments. This surprising finding presents an “area for improvement” wherein it may be possible to prevent this route of MTCT entirely by enhancing milk sIgA specificity toward a high proportion of HIV-reactive Abs using a maternal vaccination regimen targeting the gut associated lymphoid tissue (GALT), the origin tissue of the B cells that ultimately produce milk sIgA (known as the entero-mammary link). NHPs, particularly Rhesus Macaques (RMs), are an obvious choice for pre-clinical work in this area, given their extensive use in the HIV field; however, the entero-mammary link has not been verified or analyzed in NHPs. As such, it is critical to determine if an entero-mammary link exists in NHPs, and to fully describe this link prior to embarking on a targeted maternal vaccination program. This is the subject of our project in RMs that compares the sequences of immunoglobulin genes amplified from B cells isolated from GALT, respiratory mucosa, mammary tissue, milk, and blood; the reactivity profile of recombinant antibodies produced from these genes to a panel of enteric antigens; and the cellular homing receptor/adhesion marker profiles of B cells isolated from these various compartments. Should the proposed analysis demonstrate a clear link between gut and mammary B cells in RMs, we would proceed with immunogenicity and protection studies aimed to induce HIV-specific sIgA in milk via maternal vaccination targeting the GALT. We also intend to assess the entero-mammary link in pigs as a potential model for such vaccine development.
