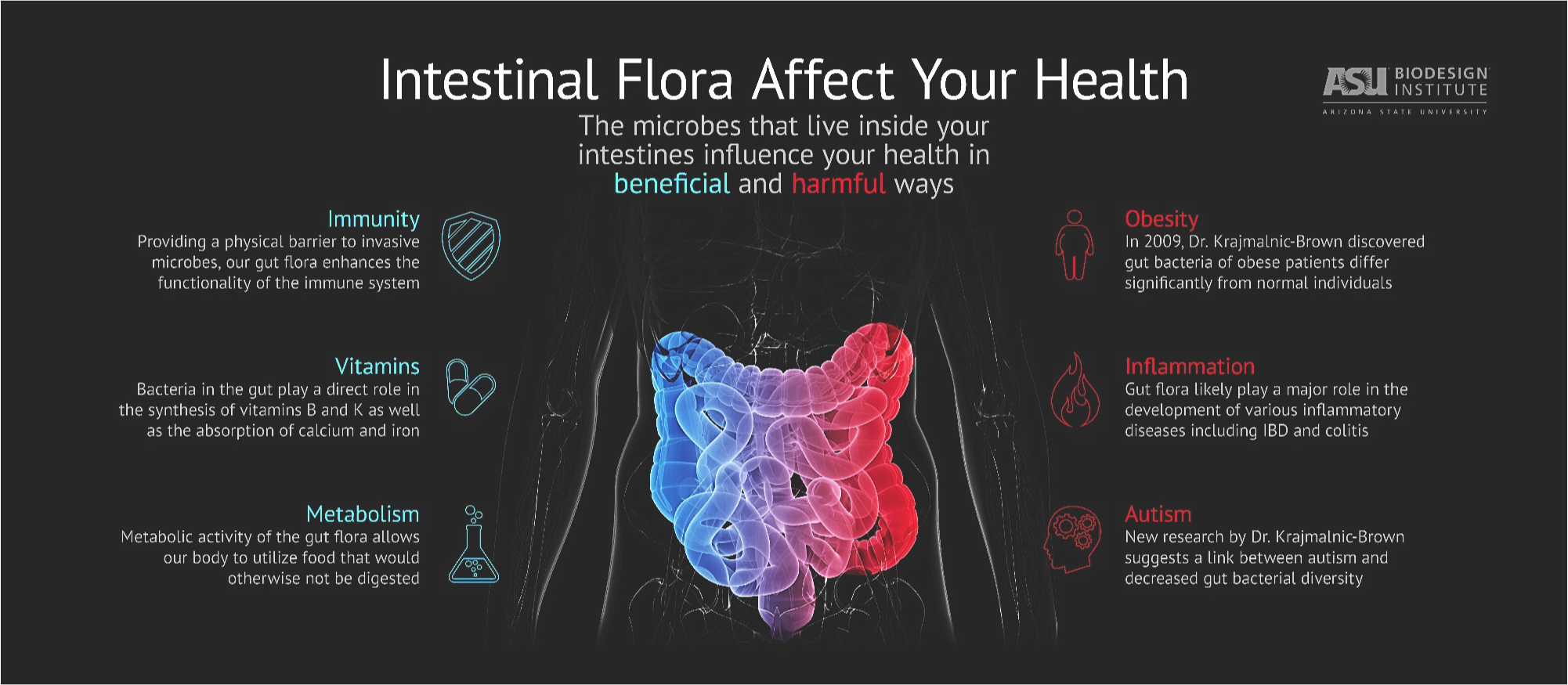
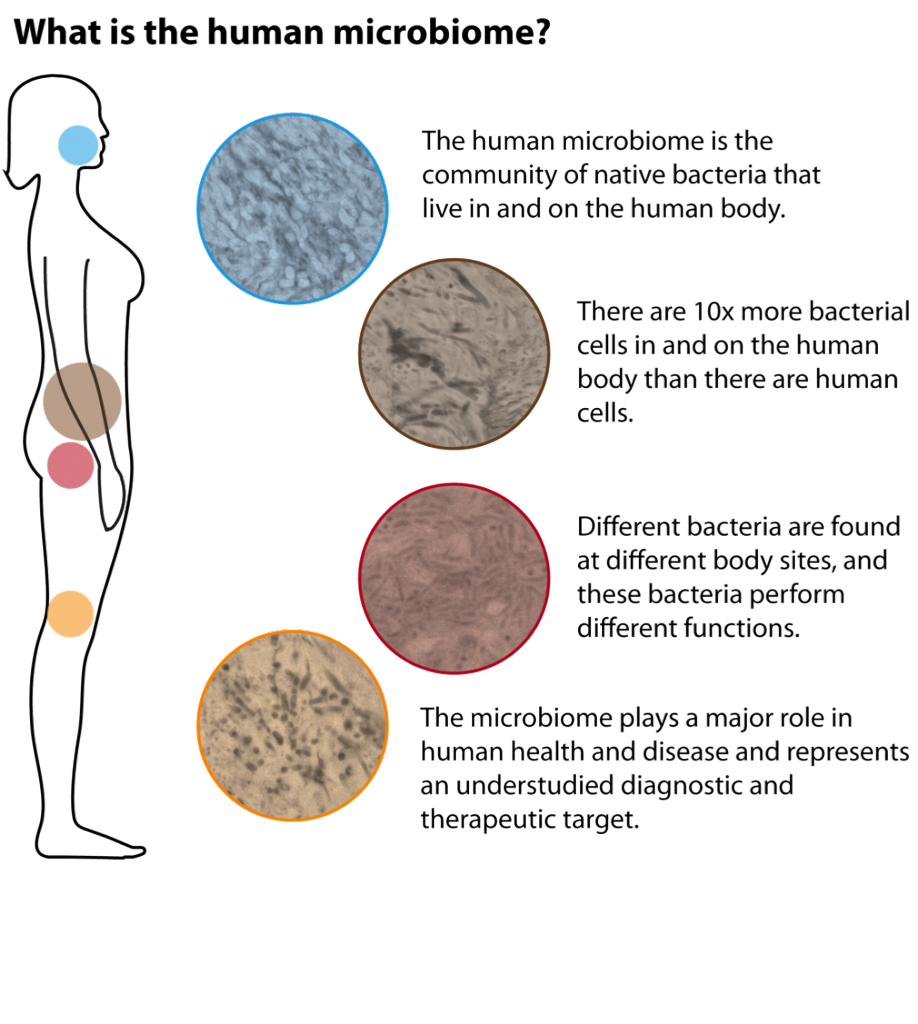
 The “microbiota” or “microbiome” is defined as all the microorganisms that cover the surface of the human body, inside and outside. This microscopic life includes thousands of species and it has been estimated that there are 10 times more microbial cells in the human body than human cells! With the advent of sophisticated technology that can measure the presence of these microorganisms, only now have researchers started to understand the huge importance of these microorganisms for human beings. For example, the gut microbiome helps to digest food, protects against harmful bacteria and trains the immune system. To be effective, the microbiome needs to be well balanced between “good” microorganisms and “bad” microorganisms. A dysregulation in this balance is called “dysbiosis” and is implicated in many diseases, such as inflammatory bowel disease, obesity, diabetes, colorectal cancer, etc.
The “microbiota” or “microbiome” is defined as all the microorganisms that cover the surface of the human body, inside and outside. This microscopic life includes thousands of species and it has been estimated that there are 10 times more microbial cells in the human body than human cells! With the advent of sophisticated technology that can measure the presence of these microorganisms, only now have researchers started to understand the huge importance of these microorganisms for human beings. For example, the gut microbiome helps to digest food, protects against harmful bacteria and trains the immune system. To be effective, the microbiome needs to be well balanced between “good” microorganisms and “bad” microorganisms. A dysregulation in this balance is called “dysbiosis” and is implicated in many diseases, such as inflammatory bowel disease, obesity, diabetes, colorectal cancer, etc.
Microbiome and IBD
Inflammatory bowel diseases (IBD) are caused by a dysregulated immune reaction to an environmental trigger in a genetically predisposed person. Recent studies have shown that IBD patients have a different microbiome compared to a non-IBD person. However, researchers still do not know if this dysbiosis is a cause or just a mere consequence of the inflammation. Many scientists have hypothesized that the microbiome could be part of the environmental triggers, mentioned above, and therefore could have a role in the cause of the disease. This dysbiosis (unbalanced microbiome) is a topic of great interest for researchers to understand more on what could cause IBD and to answer the million-dollar question: Is it possible to prevent it?
Microbiome, pregnancy, and child development
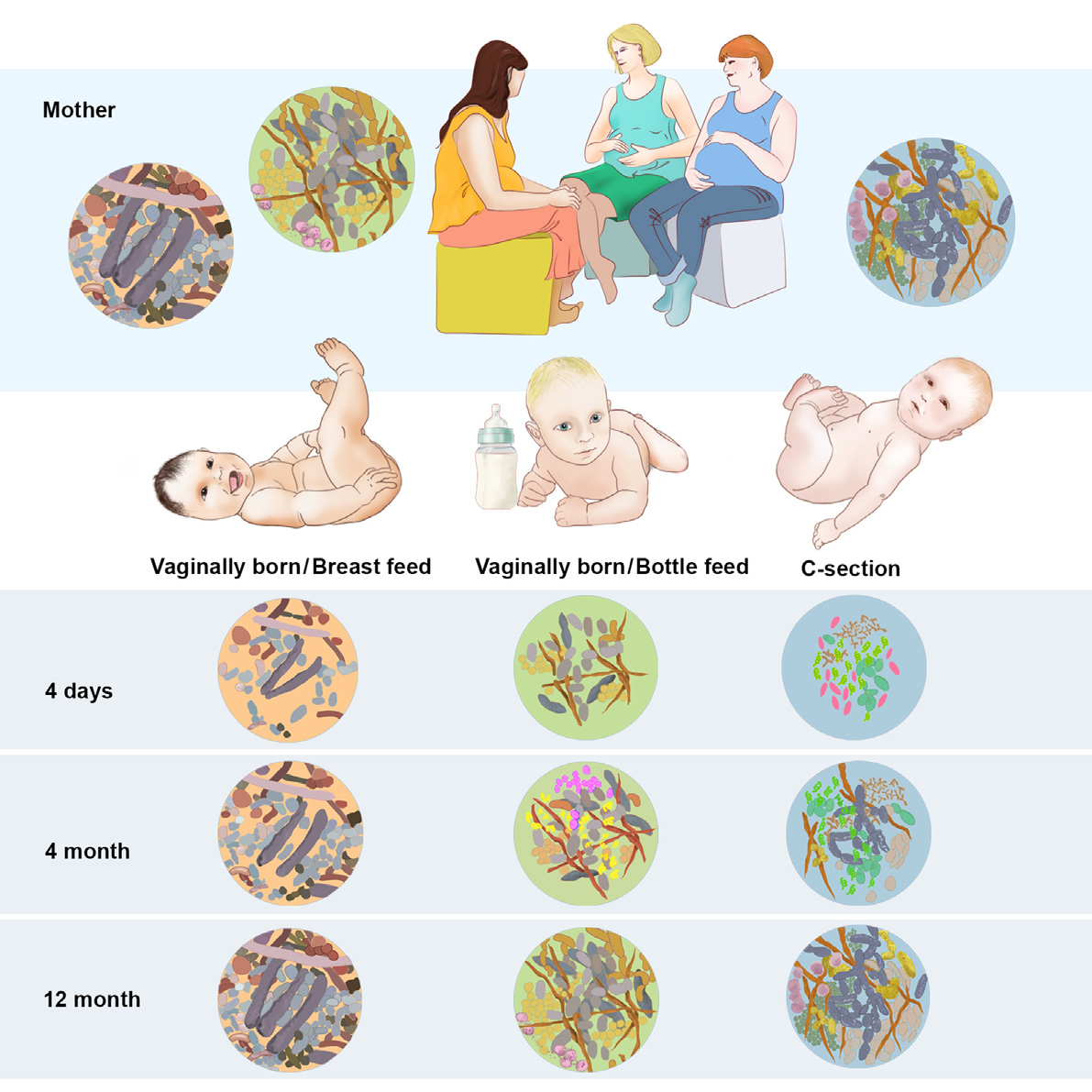
During a healthy pregnancy the body of a woman undergoes enormous changes that are mostly hormonally-driven. It is also known that in parallel with those changes many alterations occur in gut and vaginal microbiome.1 These changes could influence the microbiome of the newborn. Indeed, studies have now started to try to understand how the microbiome of the infant is formed. It has been shown, for example, that type of delivery and feeding behavior influences the baby´s gut flora in the first year of life2. It is also increasingly accepted that infant´s microbiome could be related with later development of childhood diseases such as asthma or eczema3.
Few studies have studied how maternal diseases can affect child microbiome. In a pioneer study, our group showed that babies born from mothers with diabetes had a similar bacterial composition in their meconium (the first stool that the baby passes) to their mothers, as compared to babies born from mothers without diabetes.4 This raises the possibility that the risk for acquiring some diseases could be transmitted not only through the genes, but also through the microbes. If this would prove to be true, preventative interventions, such as probiotics supplementation during pregnancy or childhood, could be implemented as a way of preventing later disease development.
Bibliography
- Prince AL, Antony KM, Ma J, Aagaard KM. The microbiome and development: a mother’s perspective. Semin Reprod Med 2014;32(1):14–22.
- Bäckhed F, Roswall J, Peng Y, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015;17(6):852.
- Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 2015;7(307):307ra152.
- Hu J, Nomura Y, Bashir A, et al. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS ONE 2013;8(11):e78257.