Viruses and their hosts are engaged in a constant struggle, much of which happens at the as-yet-not-fully-understood transcriptome level. Approximately 95% of the human transcriptome consists of non-coding RNAs (ncRNAs), and ncRNAs modulate almost every cellular process, serving as essential regulators of protein-coding messenger RNA (mRNA) levels. Host ncRNAs can play antiviral roles, but viruses also encode their own ncRNAs to exploit cellular regulatory pathways. As viral ncRNAs can significantly impact host gene expression, it is important to understand the molecular mechanisms of the interplay between viral and host transcripts in order to develop novel therapeutics for viral infections and associated diseases.
microRNAs (miRNAs) are small ncRNAs involved in post-transcriptional regulation of gene expression in animals and plants. miRNAs are loaded onto Argonaute proteins — core components of the RNA interference pathway — to guide them to partially complementary sequences on messenger RNAs (mRNAs). miRNA binding results in translation inhibition and/or mRNA decay by decapping and deadenylation. In humans, most mRNAs are regulated by miRNAs, and aberrant miRNA levels are linked to disease.
PROJECTS:
1. Understanding how ncRNAs from oncogenic herpesviruses regulate host gene expression
γ-herpesviruses are large double-stranded DNA viruses characterized by two phases: lytic, during which the productive viral replication takes place, and latent, when only few genes that induce host cell immortalization are expressed. These viral genes produce several proteins and multiple ncRNAs. Viral latency is associated with oncogenesis. 1% of human cancers, such as Burkitt’s lymphoma, Hodgkin’s disease and nasopharyngeal carcinoma, are thought to be associated with Epstein-Barr virus (EBV) infection. Also, primate herpesvirus saimiri (HVS) causes aggressive T-cell lymphomas in New World primates and can transform human T cells.
During its latency, HVS expresses seven small nuclear RNAs, known as HSUR1 through 7. HSUR1 induces degradation of a host miRNA, miR-27a, which in T cells results in prolonged cell activation that contributes to oncogenic transformation. HSUR2 selectively targets host pro-apoptotic mRNAs by mediating their interactions with miRNA-associated decay machinery.
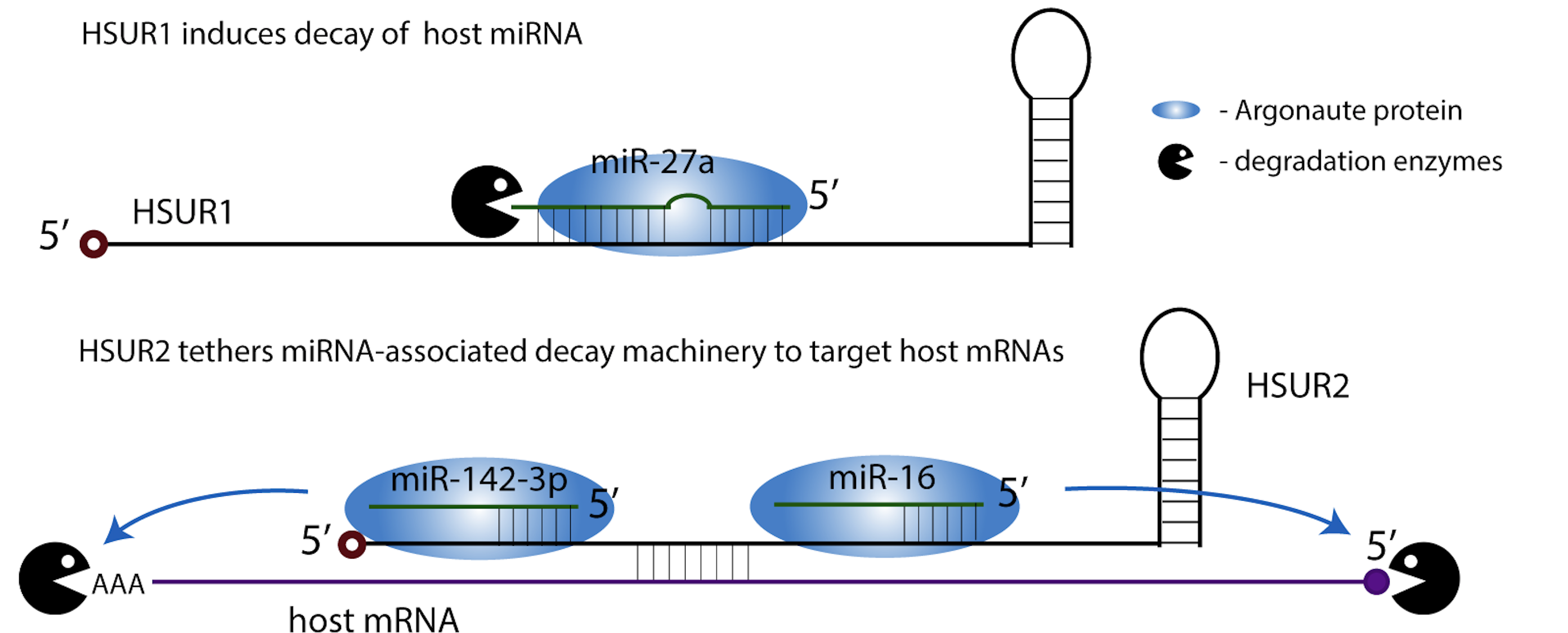
Additional mechanisms by which HSURs regulate host gene expression are waiting to be discovered!
2. Uncovering the roles of small ncRNAs in SARS-CoV-2 infection
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease (COVID-19), continues to be a pressing health concern.
We recently discovered a viral miRNA-like small RNA, named vmiR-5p (for viral miRNA), derived from the SARS-CoV-2 ORF7a transcript. vmiR-5p functionally associates with Argonaute proteins leading to downregulation of host transcripts. vmiR-5p production relies on cellular machinery, yet is independent of Drosha protein, and is enhanced by the presence of a strong and evolutionarily conserved hairpin formed within the ORF7a sequence.
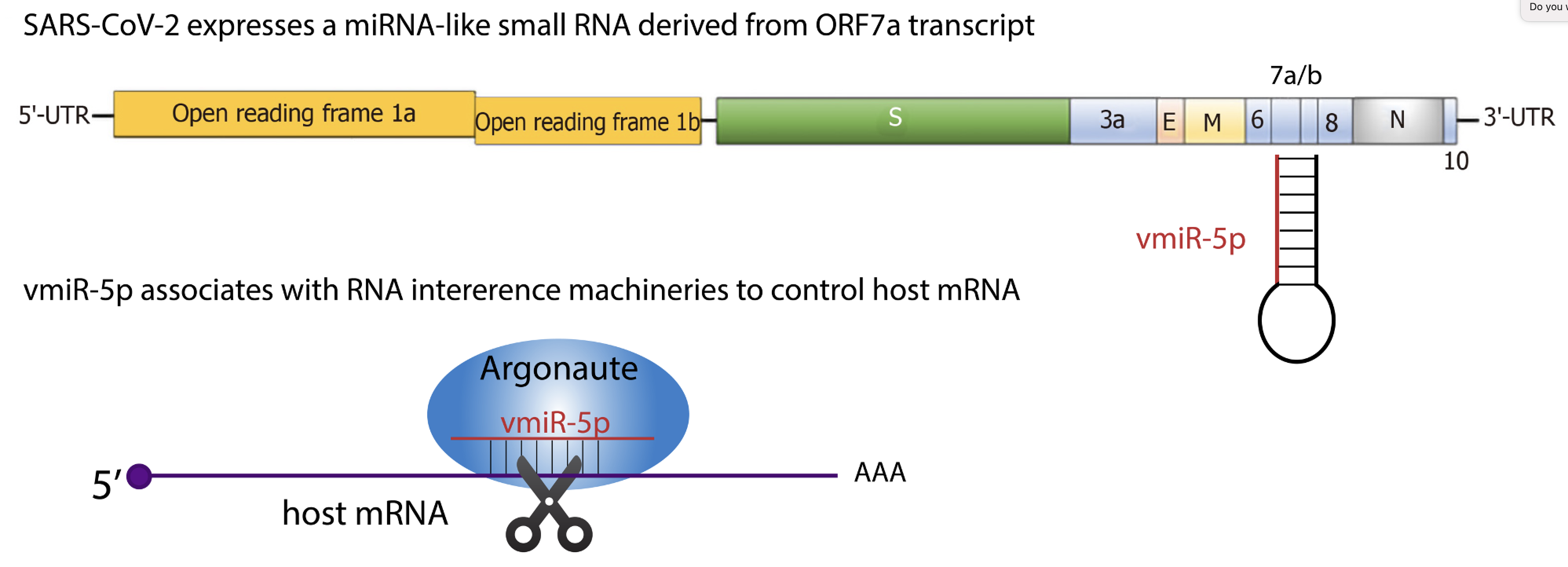
Many questions remain unanswered!
3. Deciphering the novel mechanism of host microRNA regulation by herpesviruses
Viruses evolved multiple mechanisms to regulate host gene expression; including manipulation of host miRNA levels. For example, some herpesviral transcripts elicit selective host miRNA decay in a process called target-directed miRNA degradation (TDMD). Also poxvirus poly(A) polymerase induces a widespread host miRNA polyadenylation, which leads to miRNA decay.
We have observed a dramatic decrease in many host tumor suppressive miRNAs upon latent herpesviral infection. Our preliminary data suggest the existence of an unknown mechanism by which herpesviruses dampen host miRNAs.
