Germline functions: from stem cells to fertility
Every organism starts out as a single fertilized cell, yet we do not fully understand the events that are essential for producing that cell because they take place within the ovary of the mother. Failure to form an egg that is capable of embryonic development can result in profound birth defects or miscarriage. In addition, cancers of the ovary can arise from uncontrolled proliferation of the germ cells, those cells that can become eggs, or the somatic cells, the cells that do not develop as eggs, of the ovary. In normal ovaries, these two types of cells communicate with one another to regulate the growth and survival of both cell populations. In most animals, the germ line stem cells undergo an asymmetric division to generate daughter cells that will remain stem cells and others, cystoblasts that divide and eventually form eggs. The divisions of the cystoblasts are unique because the cells do not completely separate from one another, but instead remain attached to each other. Studies in mammals show that the connections between cystoblasts prevent too many cells from becoming oocytes, and in humans uncontrolled and complete separation of cystoblasts has been correlated with germ cell neoplasias. However, since these events occur before or at the time of fertilization, we understand little about how the genes that are involved. Therefore, understanding how the growth and survival of these cells is regulated has important consequences to both fertility and cancer formation.
To study relationships between interacting cells within adjacent tissues, such as germline and somatic follicle cells, we need to analyze an animal system in which we can manipulate genes and study early development. The zebrafish system has advantages that allow us to use embryological, biochemical, and genetic techniques to access maternally controlled processes during vertebrate animal development. Our studies exploit the powerful genetics and cell biological access in the zebrafish system to unravel the mechanisms that regulate oocyte polarization and follicle cell fate in a vertebrate. Several features of primary oocyte development are common among insects, and vertebrates, including humans, thus this architecture is likely fundamental for germline development and fertility.

Contact Us
Marlow Laboratory
Florence L. Marlow, Ph.D.
Associate Professor
Department of Cell, Developmental, & Regenerative Biology
Black Family Stem Cell Institute
Mindich Child Health and Development Institute
Current Projects
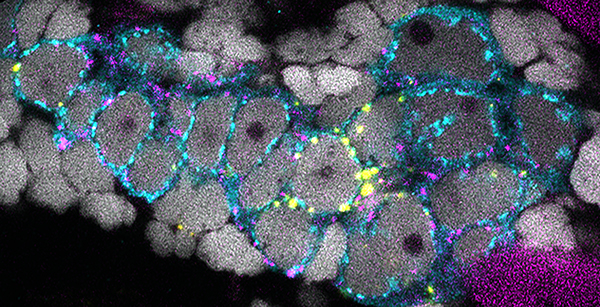
Germline stem cells and fertility
Germline stem cells are essential for fertility. Our long-term goal is to understand the mechanisms that regulate specification and maintenance of the stem cell pool. We are investigating the function and contribution of conserved genes to stem cell specification using genetic and cell biological approaches to analyze the specialized cell divisions of germline stem cells and their immediate daughters that generate germline cysts. which has implications for premature ovarian insufficiency, polycystic ovary syndrome, cancer, and fertility.
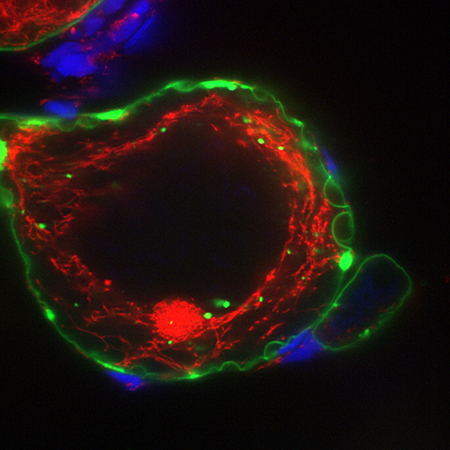
Oocyte polarity and maternal control of early development
Maternally provided gene products regulate the earliest events of embryonic life, including formation of the oocyte that will develop into an egg, and eventually an embryo; yet studies of maternal gene functions remain limited. Our goal is to develop efficient genetic methods that can be applied to identify these regulators and fill the large gaps in our understanding of the mechanisms and molecular pathways contributing to fertility and early development. Oocytes are large, highly polarized gametes that can generate all of the cell types of the organism. We apply biochemistry and maternal-effect molecular genetics to test novel hypotheses, and to decipher the mechanisms that establish conserved oocyte asymmetries and the vertebrate oocyte animal-vegetal axis. An improved understanding of the maternal genes that are crucial for polarity and patterning of oocytes, eggs, and embryonic development has implications for fertility, and will provide insight into the basis of birth defects, miscarriage, and infertility.
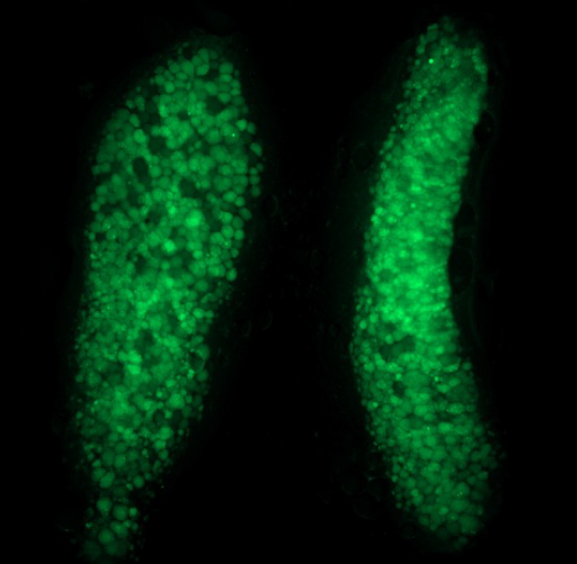
Molecular genetic basis of sex-specific differentiation of germ cells and maintenance of ovarian fates
Germline stem cells are essential for fertility. In most animals, including mammals, embryos initially develop female-like gonads that in males are remodeled into testis in males or further differentiated to form the mature ovary in females. Our long-term goal is to understand the mechanisms that regulate specification and maintenance of the germline, which are essential for reproductive health and fertility. Deficits in sex determination can lead to Disorders of Sexual Development and infertility. We investigate the function and contribution of conserved genes to plasticity and sex-specific differentiation of germ cells and secondary sex traits. This work will take advantage of the powerful zebrafish genetic tools to study conserved cell types and genes to understand their contributions to normal gonad development and also to female reproductive system diseases, including premature ovarian insufficiency, polycystic ovary syndrome, fertility, and cancer, areas of biomedicine greatly in need of more research.
Microglia
We are using genetic models to investigate sexual dimorphism in microglia and their involvement in shaping neural circuits underlying complex behaviors in juveniles and adults.
Recent Publications
Bertho S, Kaufman OH, Lee KL, Santos-Ledo A, Dellal D, Marlow FL. A transgenic system for targeted ablation of reproductive and maternal-effect genes. Development. 2021 148(12):dev198010.doin:10.1242/dev.198010.PMID:34143203 PMCID:PMC8254866.
Bertho S, Clapp M, Banisch T, Bandemer J, Raz E, Marlow FL. Zebrafish dazl regulates cystogenesis and germline stem cell specification during the primordial germ cell to germline stem cell transition. Development. 2021 148(7):dev187773. doin:10.1242/dev187773. PMID:33722898 PMCID: PMC8077517.
Romano S, Kaufman OH, Marlow FL. Loss of dmrt1 restores zebrafish female fates in the absence of cycp19a1a but not rbpms2a/b. Development. 2020 147(18):dev190942/dev.190942. PMID 32895289 PMCID: PMC7541348.
Kaufman OH, Lee K, Martin M, Rothhämel S, Marlow FL. Zebrafish rbpms2 functions in Balbiani body architecture and oocyte fate. PLoS Genetics. 2018 14(7): e1007489. https://doi.org/10.1371/journal.pgen.1007489.
Santos-Ledo A, Garcia-Macia M, Campbell PD, Gronska M, Marlow FL. Kinesin 1 promotes chondrocyte maintenance during skeletal morphogenesis. PLoS Genetics. 2017 13(7):e1006918. doi.org/10.1371/journal.pgen.1006918. PMID: 29140986.
Selected Reviews
Marlow FL. Setting up for Gastrulation in Zebrafish. Gastrulation. Edited by Lilianna Solnica-Krezel. Elsevier. Current Topics in Developmental Biology. 2020 Vol 136 pp 33-83 https://doi.org/10.1016/bs.ctdb.2019.08.002
Lee K and Marlow FL. Visualizing the Balbiani Body in Zebrafish Oocytes. Francisco J. Pelegri (ed.), Vertebrate Embryogenesis: Embryological, Cellular, and Genetic Methods, Methods in Molecular Biology, vol. 1920, https://doi.org/10.1007/978-1-4939-9009-2_16, © Springer Science+Business Media, LLC, part of Springer Nature 2019.
Marlow FL. Recent advances in understanding oogenesis: Interactions with the cytoskeleton, microtubule organization, and meiotic spindle assembly in oocytes. F1000Research 2018, 7(F1000 Faculty Rev):468 (doi: 10.12688/f1000research.13837.1).
Clapp M. and Marlow FL. Acquisition of oocyte polarity. Results and Problems in Cell Differentiation. 2017;63:71-102. doi: 10.1007/978-3-319-60855-6_4. PMID:28779314.
Marlow FL. Mitochondrial matters: Mitochondrial bottlenecks, self-assembling structures, and entrapment in the female germline. Stem Cell Research. 2017 May;21:178-186. doi: 10.1016/j.scr.2017.03.004. Epub 2017 Mar 15. PMID: 28336253.
Marlow FL. Primordial Germ Cell Specification and Migration. F1000Research 2015, 4(F1000 Faculty Rev):1462 (doi: 10.12688/f1000research.6995.1).
Team

 Paloma Bravo MS
Paloma Bravo MS
Research Assistant/Student
paloma.bravo@mssm.edu
 Maya Pahima
Maya Pahima
PhD Student
maya.pahima@icahn.mssm.edu
 Gia Tolins
Gia Tolins
PhD Student
gia.tolins@icahn.mssm.edu
 Miranda Wilson
Miranda Wilson
PhD Student
miranda.wilson@icahn.mssm.edu
 Auset Taylor
Auset Taylor
Education Coordinator Bioeyes NYC
auset.taylor@mssm.edu

Sylvain Bertho PhD
Postdoctoral Fellow
sylvain.bertho@mssm.edu

Shannon Romano PhD
Postdoctoral Fellow
shannon.romano@mssm.edu

Paloma Bravo Correa
Research Assistant
paloma.bravo@mssm.edu
Alumni
Graduate Students
- Odelya H. Kaufman (MD/PhD)
- Philip Campbell (MD/PhD)
- Xin Lee (PhD)
Postdoctoral Fellows
- Devora Aharon MD
- Sylvain Bertho PhD
- Shannon Romano PhD
- Adrian Santos-Ledo PhD
- ALexa Clapp MD
- Lei Feng PhD
- Andreas Zaucker PhD
- David Aphkhazava PhD
- Sophie Rothhamel DPhil
Masters Students
- Nitya Khatri
- Clara DeLaroque
- Manon Budzyk
- Kim Westerich
- Manon Martin
- Cindy Guilaume
- Elodie Ferreira
- Raissa Sedaminou Zossou
- Hannah Perpetua
- Lydia Djenoune
Research Assistants
- KathyAnn Lee
- Amanda Heim
Lab News
So excited to welcome PhD Student Gia Tolins to the lab 🙂
Links of interest
ZFIN
https://zfin.org
IZFS
https://www.izfs.org
ZIRC
https://zebrafish.org/home
SDB
https://www.sdbonline.org
Location
Annenberg 2584
Phone: 212-241-5067
Office: 212-241-5067
Lab: 212-241-4160
florence.marlow@mssm.edu
