Research
Extreme stress and/or fear can profoundly affect brain and body mechanisms leading to increased vulnerability for developing disorders such as post-traumatic stress (PTSD) and panic disorders. Stress exerts its effects on the brain and body via the sympathetic and parasympathetic systems which allow organisms to tackle a variety of stressors with “fight or flight” responses regulated by the sympathetic nervous system (SNS), and “rest and digest” responses, regulated by the parasympathetic nervous system (PNS). Extreme or prolonged activation of the sympathetic systems can have detrimental effects at the level of neurons and astrocytes in various brain regions, neurotransmitter and neuropeptidergic systems, and organs like heart, lungs and gut in turn affecting central, metabolic, cardiorespiratory and immune functions. The vagus nerve, a major cranial nerve connecting the brain and body and known as the “wandering nerve”, is an important regulator of parasympathetic functions via innervations to the thoracic and abdominal organs, and the autonomic, cardiovascular, respiratory, gastrointestinal, immune, and endocrine systems. Direct vagus nerve stimulation using electrical devices or through mind/body practices such as yoga appear to be effective in diminishing stress, immune, and cardiorespiratory functions.
Karki lab is dedicated to understanding the reciprocal mechanisms between the brain and body axis via the sympathetic and parasympathetic modulators that affect or are affected by traumatic stress and associated changes in cardiorespiratory functions. Our research questions span along three main areas: 1) How is the brain-vagus-body axis involved in modulating fear and stress? 2) What are the mechanisms that link traumatic stress and metabolic syndromes?, and, 3) How are brain astrocytes involved in regulating fear, stress and cardiorespiratory functions? Our work is focused on the neuropeptidergic systems like PACAP (pituitary adenylate cyclase activating peptide) and CRF (corticotropin releasing factor) and the neurotransmitter norepinephrine. To answer our questions, we use cutting-edge tools and techniques such as optogenetics, chemogenetics, genetically modified mouse lines, intersectional viral approach, telemetry tracking of cardiorespiratory functions, behavioral models of traumatic stress and fear, and many others.
Contact Us
Karki Laboratory
Abha Karki Rajbhandari
Principal Investigator
abha.rajbhandari@mssm.edu
Current Projects
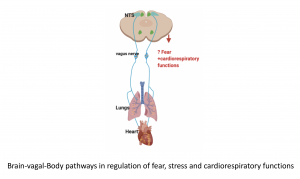
1. How is the brain-vagus-body axis involved in modulating fear and stress?
This projects seeks to investigate the role of reciprocal connections between the brain and body (heart and lungs) via the vagus nerve in regulating traumatic fear, stress and cardiorespiratory functions.
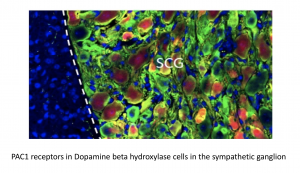
2. What are the mechanisms that link traumatic stress and metabolic syndromes?
This project investigates the link between traumatic stress and metabolic changes with relevance to obesity, diabetes and other metabolic syndromes in stress.
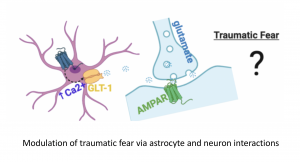
3. How are brain astrocytes involved in regulating fear and stress?
This project is investigating the role of brain astrocytes and their modulation by neuropeptides in regulation of fear, stress and cardiorespiratory functions.
Funding & Awards
Current Funding support:
2023-2027 Department of Defense Investigator Initiated Research Award
2022-2024 R21- National Institute of Diabetes and Digestive and Kidney Diseases
2020-2023 Brain and Behavior Research Foundation NARSAD Young Investigator Grant
2020 Whitehall Foundation Grant
2020 Akira Arimura Young Investigator Grant for VIP/PACAP Research
2020 Friedman Brain Institute Scholars Award
Honors:
2023 Invited Keynote Speaker at the Bronx County Science Fair
2022 Distinguished Scholar Award for grant writing
2019 Arimura Award for Young Investigators for Excellence in VIP/PACAP Research
2018 Brain and Behavior Research Foundation NARSAD Young Investigator Award
2015 Ruth L. Kirschstein National Research Service Award (NRSA) Individual Postdoctoral Fellowship
2015 Trainee Professional Development Award, Society of Neuroscience
2015 Brain Research Institute Travel Award for Society of Neuroscience, UCLA
2012 Vilas Conference Grant for Society for Neuroscience Conference
2011 Competitive Travel Award (Neuroscience Training Program)
2010 Competitive Training Grant-T32 GM007507
2006 Social Science award, Olivet College
2004-2006: President’s List, Olivet College
2004-2006: Dean’s List, Olivet College
2004-2005: Heritage grant, Olivet College
2004-2005: S-Varney Scholarship, Olivet College
Recent Publications
PEER-REVIEWED JOURNAL ARTICLES
- Patel S, Sparman NZR, Arneson D, Alvarsson A , Santos LC, Duesman SJ, Centonze A, Hathaway E, Ahn IS, Diamante G, Cely I, Cho CH, Talari NK, Rajbhandari AK, Goedeke L, Wang P, Butte AJ, Blanpain C, Krishnan KC, Lusis AJ, Stanley SA, Yang X, Rajbhandari P. Mammary duct luminal epithelium controls cold-induced adipocyte thermogenic program 2023. Nature. In Press.
- Van C, Condro MC, Hrncir H, Diep AL, Rajbhandari AK, Fanselow M, Hashimoto H, Mackenzie-Graham AJ, Waschek JA. Overexpression of VIPR2 in mice results in microencephaly with paradoxical increased white matter volume. 2023.Experimental Neurology.
- Khandelwal A, Cushman J, Wazir S, Choi J, Zhuravka I, Rajbhandari AK*, Valiulahi P, Li X, Zhou C, Comai L*, Sita Reddy1*. Mbnl2 inactivation alters cognition of novel spatial frameworks to impair object recognition memory. iScience. 2023.
- Duesman SJ, Shetty S, Patel S, Ogale N, Mohamed F, Sparman N, Rajbhandari P, Rajbhandari AK. Sexually dimorphic role of the locus coeruleus PAC1 receptors in regulating acute stress-associated energy metabolism. 2022. Frontiers in Behavioral Neuroscience.
- Rajbhandari AK , Octeau JC, Gonzalez S, Pennington ZT, Trott J, Chavez J, Ngyuen E, Keces N, Hong WZ, Neve RL, Waschek J, Khakh BS, Fanselow MS. A basomedial amygdala to intercalated cells microcircuit expressing PACAP and its receptor PAC1 in contextual fear regulation. 2021. Journal of Neuroscience. -Corresponding author
- Rajbhandari P, Arneson D, Feng AC, Ahn IS, Diamante G, Zaghari N , Thomas BJ , Vergnes L , Lee SD , Rajbhandari AK*, Reue K, Smale ST, Yang X and Tontonoz P. Single Cell Analysis Reveals Immune Cell-Adipocyte Crosstalk Regulating the Transcription of Thermogenic Adipocytes. 2019. Elife.
- Rajbhandari AK, Bakshi VP. Repeated norepinephrine receptor stimulation in the BNST induces sensorimotor gating deficits via corticotropin releasing factor. 2020. Neuropharmacology.
- Pennington ZT, Trott, JM, Rajbhandari AK, Li K, Walwyn WM, Evans CJ, Fanselow MS. Chronic opioid pretreatment potentiates the sensitization of fear learning by trauma. 2019. Neuropsychopharmacology.
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Sotiris MC, Fanselow MS, Khakh BS. Astrocytes induce acute hyperactivity in adult mice by reactivation of a latent synaptogenic cue. 2019.
- Rajbhandari AK, Gonzalez ST, Fanselow MS. Stress-Enhanced Fear Learning, a Robust Rodent Model of Post-Traumatic Stress Disorder. Journal of Visualized Experiments.
- Angarita S, Truong B, Khoja S, Lin MG, Lam AK, Nitzahn M, Rajbhandari AK, Zhuravka I, Duarte S, Cederbaum SD, Lipshutz GS. Human Hepatocyte Transplantation Corrects the Inherited Metabolic Liver Disorder Arginase Deficiency in Mice. Molecular Genetics and Metabolism.
- Ago Y, Hayata-Takano A, Kawanai T, Yamauchi R, Takeuchi S, Cushman JD, Rajbhandari AK, Fanselow MS, Hashimoto H, Waschek JA. Impaired extinction of cued fear memory and abnormal dendritic morphology in the prelimbic and infralimbic cortices in VPAC2 receptor (VIPR2)-deficient mice. Neurobiology of Learning and memory. 145: 222-231
- Rajbhandari AK, Zhu R, Adling C, Fanselow MS, Waschek JA. 2016. Graded fear generalization enhances the level of cfos-positive neurons specifically in the basolateral amygdala. Journal of Neuroscience Research. 94(12):1393-133
- Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Van C, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek J. High-resolution characterization of a PACAP-EGFP transgenic mouse model: Mapping the circuitry of PACAP-expressing neurons. Journal of Comparative Neurobiology. 524(18):3287-3848
- Rajbhandari AK, Bakshi VP. 2015. Trauma-induced CRF release causes lasting neuroadaptations in basolateral amygdala norepinephrine systems that promote PTSD-like startle abnormalities. Journal of Neuroscience. 35(42):14270-85 (*Press release of paper at Milwaukee, WI Journal Sentinal and UW-Madison).
- Ago Y, Condro MC, Rajbhandari AK, Van C, Jayaram B, May V, Waschek J. 2016. PACAP modulation of CNS and peripheral inflammation. PACAP-Current Topics in Neurotoxicity, vol 11 pp651-670
- Alsene KM*, Rajbhandari AK*, Ramaker MJ, and Bakshi VP. 2011. Discrete Forebrain Neuronal Networks Supporting Noradrenergic Regulation of Sensorimotor Gating. Neuropsychopharmacology. *=co-first authors. 36(5):1003-14
- Baisley SK, Fallace KL, Rajbhandari AK, Bakshi VP. 2011. Mutual independence of 5-HT2 and α1 noradrenergic receptors in mediating deficits in sensorimotor gating. Psychopharmacology (Berl). 220(3):465-79
- Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. 2010. Dopamine receptor modulation of repetitive grooming actions in the rat: Potential relevance for Tourette syndrome. Brain Research. 1322:92-101
-
REVIEW ARTICLES
- Nishimura KJ, Poulos A, Drew M, Rajbhandari AK. Know thy SEFL: Fear sensitization and its relevance to stressor-related disorders. 2022. Neuroscience and Biobehavioral Reviews.
- Rajbhandari AK. Aversive Memory Storage in the Basolateral Amygdala: The Nut May Be Cracked. 2022. Biological Psychiatry
- Rajbhandari AK, Tribble J, Fanselow MS. 2017. Neural circuits of fear memory. Learning and Memory: A Comprehensive Reference 2e.
-
-
PRESS COVERAGE
1. 2019 Rajbhandari AK. UCLA Clinical and Translational Science Institute. Arimura Award for Young Investigators for Excellence in VIP/PACAP Research. https://ctsi.ucla.edu/news/item?item_id=780999
2. 2015 Rajbhandari AK, Bakshi VP. 2015. Trauma-induced CRF release causes lasting neuroadaptations in basolateral amygdala norepinephrine systems that promote PTSD-like startle abnormalities. (*Press release of paper at Milwaukee, WI Journal Sentinal). http://archive.jsonline.com/blogs/news/351397551.html
Team

Abha Karki Rajbhandari, Ph.D.
Principal Investigator
abha.rajbhandari@mssm.edu

Benjamin Keller
Research Associate

Samuel Duesman, Ph.D.
Postdoctoral Researcher

Pamela Toh
Masters Student

Sanutha Shetty, Masters in Biomedical Science
Neuroscience PhD Graduate Student

Gabriela Picazo
Ceter for Youth Excellence Student

Andrew Fajardo
Masters Student
Alumni
Anika Das, Graduate Student (Currently University of Michigan)
Neha Ogale, Research Associate (Currently Research Coordinator at NYU)
Shade Eleazer, Research Assistant (Currently Graduate student at Yale University)
Kennedy Blankenship, Research Volunteer.
Farzanna Mohamed, Research Associate. (Currently Graduate Student at University of Michigan)
Sydney Hart, Research Associate. (Currently Graduate Student at NYU)
Positions Available
Current available positions
We are looking for a graduate student to join the lab. Pease email abha.rajbhandari@mssm.edu
Contact Us
Abha Karki Rajbhandari
Principal Investigator
abha.rajbhandari@mssm.edu
