Finding the Best Combination of Brain and Spinal Cord Stimulation with Hand Training after Spinal Cord Injury
Background
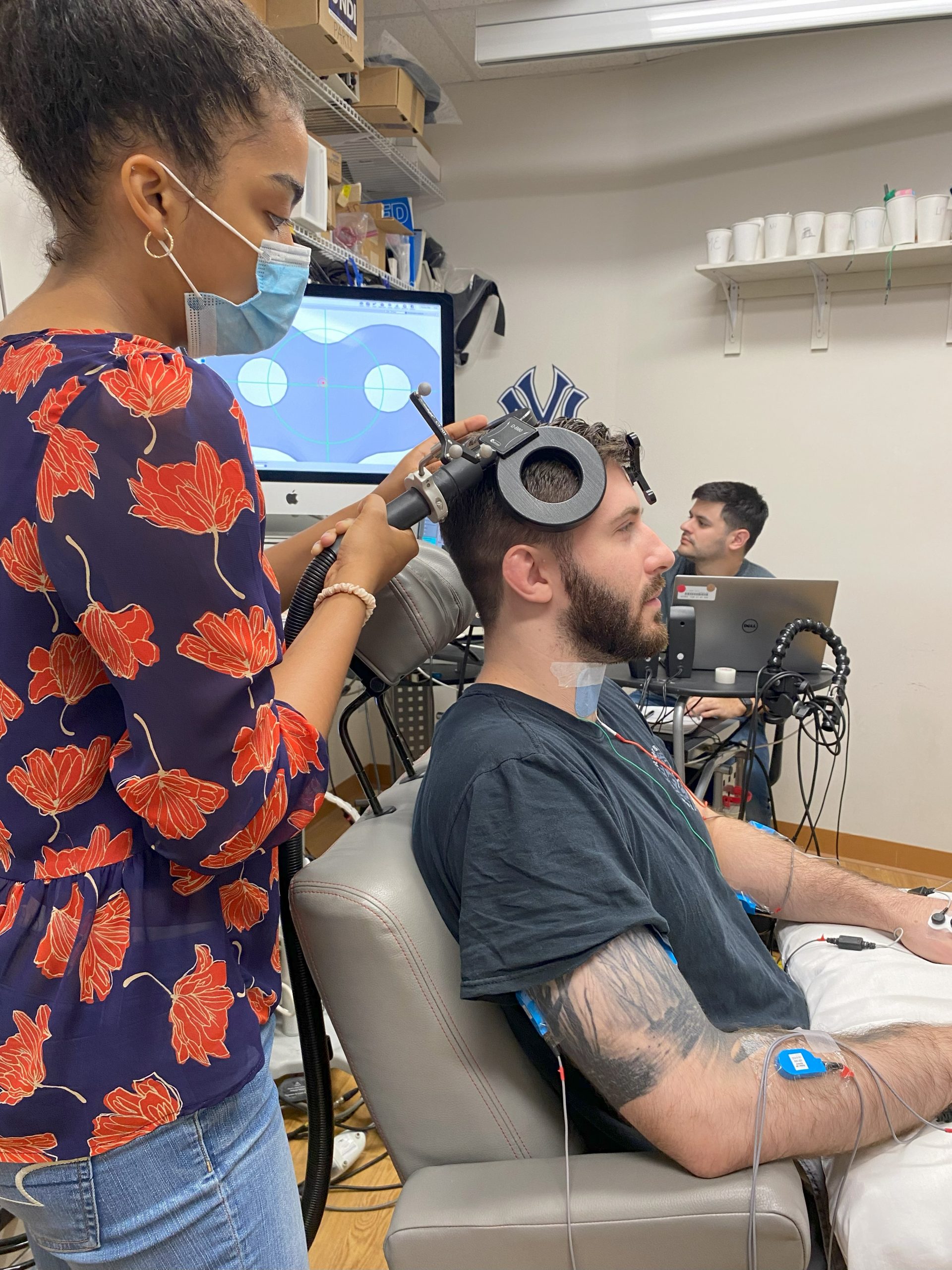
This is a follow-up of the ‘Spinal Cord Associative Plasticity (SCAP)’ study. In people with chronic
cervical SCI, we will systematically test parameters of brain and cervical spinal cord stimulation such as pulse frequency, number of bouts of SCAP per session, time interval between bouts of SCAP, and the effect of combining SCAP with functional hand exercises. The goal is to determine each participant’s optimal individualized method for delivering repetitive SCAP along with functional hand exercises to obtain a more lasting effect of at least 24 hours.
Note: This is a research study testing for temporary changes in nerve transmission and volitional motor function. There is no guarantee of long-term benefit from this study. Any benefits seen in this study will be implemented in future studies attempting to augment that effect.
Eligibility Criteria (summary)
Age 18-85 years old with chronic cervical SCI, occurred greater than 12 months ago
Ability to at least slightly move muscles of left or right hand and fingers
No history of severe traumatic brain injury (TBI); no cardiac arrhythmia; not ventilator-dependent; no implanted stimulators or cardiac pacemaker/defibrillator, no epilepsy
Other eligibility criteria will be screened in person to make sure it is safe for you to participate
Procedures
This study involves a significant time commitment: 53 visits over a period of 6-9 months.
What should you know
Electrical and magnetic stimulation can feel like a ‘shock’ that is temporarily uncomfortable at higher pulse strength
The main risk of magnetic brain stimulation is seizure. BUT:
The exclusion criteria are designed to prevent anyone at risk of seizure from participating.
When performed according to these recommendations, the risk of seizure is less than 1 in 1,000.
If you are uncomfortable at any time for any reason, we can stop the procedures. There is no obligation!!
Investigator
Noam Y. Harel, MD, PhD
Study ID
SCI SCAP+HAND
Contact
Sig Sigafose or Francisco Castano
Clinicaltrials.gov Identifier
NCT06104735