CURRENT RESEARCH PROJECTS

Slowing Down Lysosomes Revert Old Blood Stem Cells to a Youthful State; Arif*, Qiu* et al, Cell Stem Cell, 2025. Old body (blood) leaves the young body when lysosomal degradation slows down. Illustrated by: Anusree Lohithakshan, 2025.
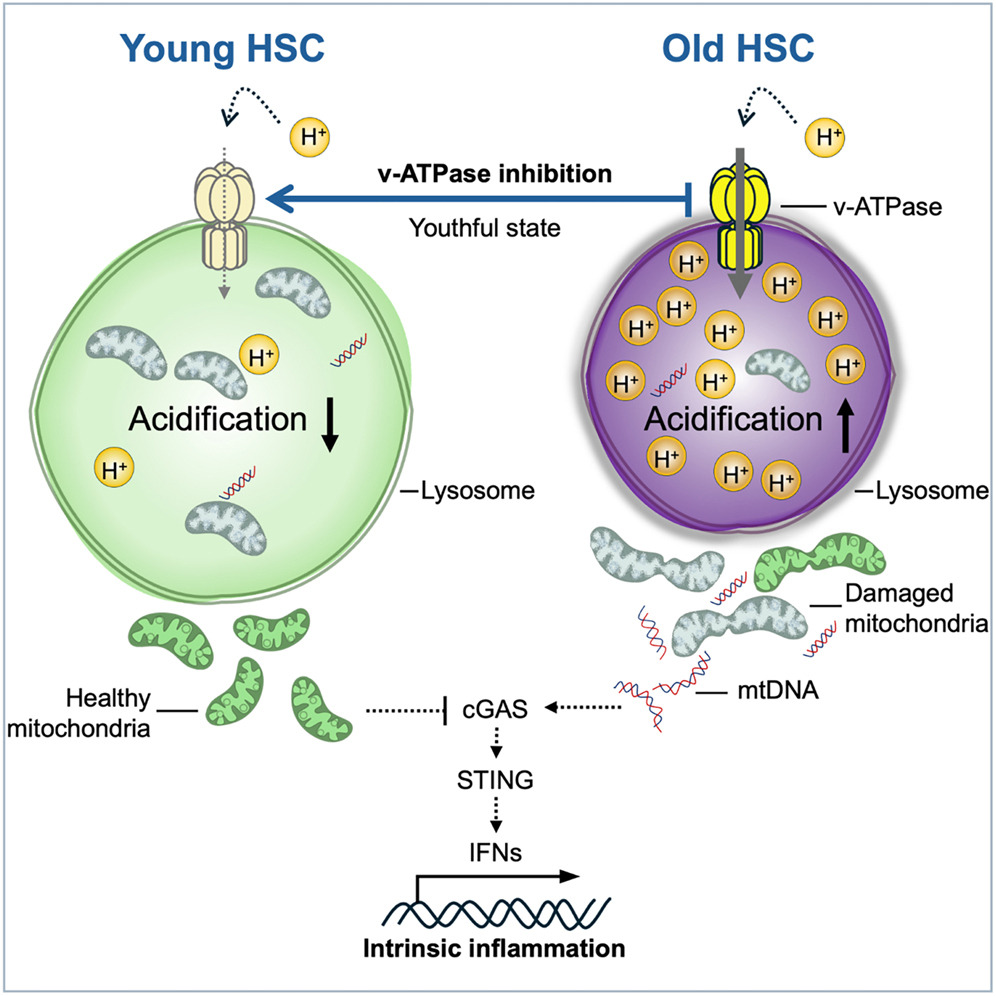
Graphical Abstract from Arif*, Qiu* et al, Cell Stem Cell, 2025.

Blood Stem Cells Hibernate via Lysosomes; Liang*, Arif* et al, Cell Stem Cell, 2020. Old yellow leaves in the Winter (the waste of hibernating quiescent blood-forming stem cells) are stored and recycled in the green bin (lysosomes) to produce the blooming tree in the Spring (primed active blood-forming stem cells). Illustrated by: Ni-Ka Ford, Katrina Kapp 2020.
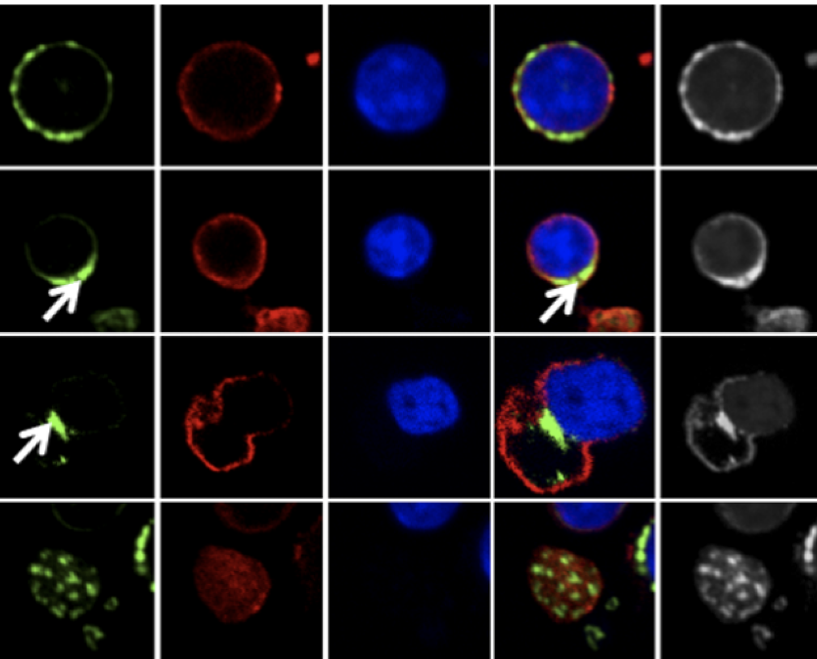
Mitochondrial positioning behind the nucleus during erythroid enucleation
(Liang et al., Blood Advances, 2021).
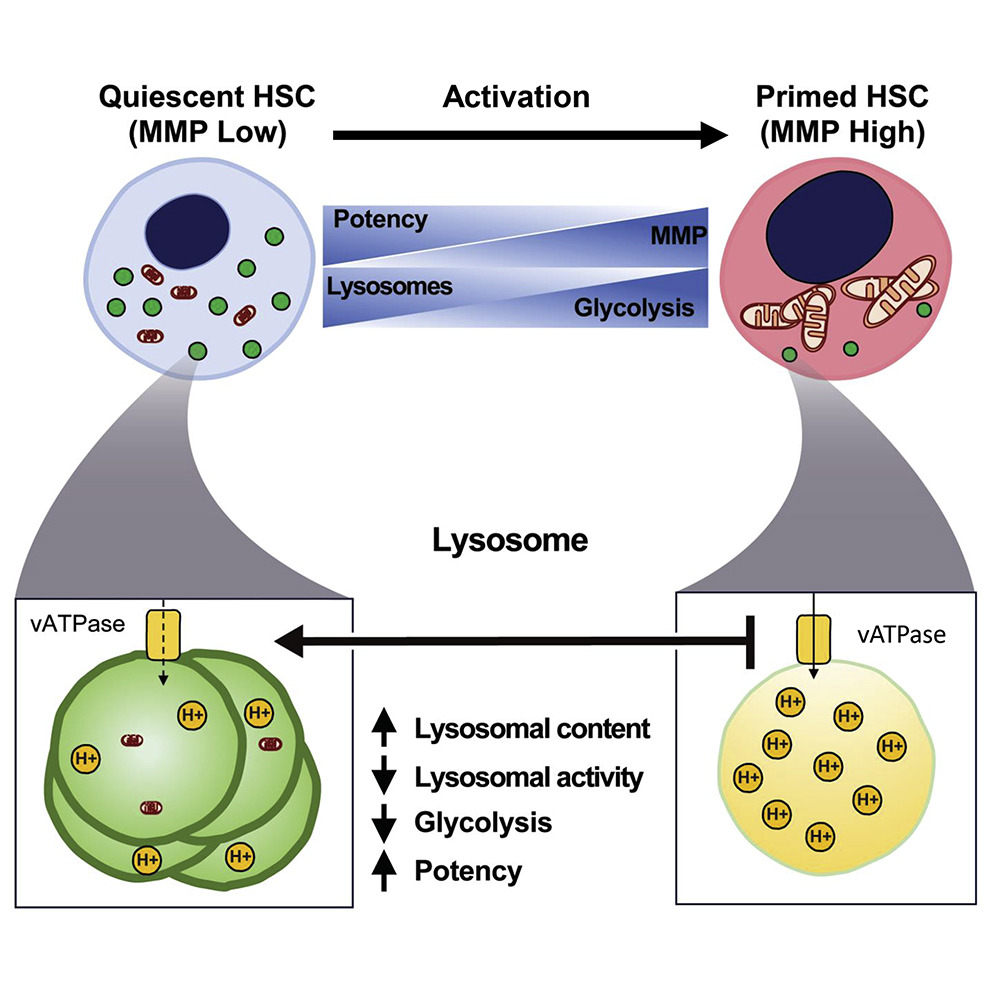
Lysosomal regulation of HSC quiescence versus priming
(Liang et al., Cell Stem Cell, 2020).
Hematopoietic Stem Cells and Their Organelles (Lysosomes, Mitochondria) Towards Improving Stem Cells’ Function, Health and Longevity
Hematopoietic stem cells (HSCs) are exceedingly rare in the bone marrow, yet they sustain the continuous production of a massive, diverse, and balanced blood system throughout life. This output is tightly regulated to meet daily physiological demands and to regenerate the blood after injury or loss, such as during hemorrhage. Central to HSC function is their ability to remain quiescent, a property that diminishes with age. Age-associated loss of quiescence and decline in HSC function contribute to immune deficiencies, imbalanced hematopoiesis, and clonal hematopoiesis. The Ghaffari laboratory investigates the mechanisms that maintain HSC quiescence and function, and how these mechanisms are disrupted in disease – particularly in blood malignancies – and during aging. We are particularly interested in elucidating how aging promotes the emergence of blood malignancies – specifically leukemia –.
One such mechanism recently uncovered by the lab – by leveraging HSCs‘ mitochondrial membrane potential – centers on lysosomes (Liang*, Arif* et al., Cell Stem Cell, 2020; Ghaffari, Cell Stem Cell, 2021; Qiu et al., STAR Protocols, 2022; Arif*, Qiu* et al., Cell Stem Cell, 2025). Lysosomes – cellular recycling centers that break down and repurpose macromolecules – play a pivotal role in metabolic regulation. Our findings reveal a core lysosomal pathway linking stem cell aging and inflammation, offering new strategies to prevent or reverse HSC aging and potentially slow or mitigate the progression of age-related blood disorders. This approach also holds promise for improving stem-cell transplantation outcomes, in older adults, and provide more effective conditions for gene therapy and may ultimately support healthier aging.
Our current work aims to elucidate how lysosomes regulate HSC fate – what are the molecules involved, how lysosomes regulate HSC metabolism – and how disruptions in lysosomal pathways contribute to blood cancers, especially leukemias. We are also developing assays to identify new modulators of lysosomal function.
Our work is also partly focused on reversible mechanisms that promote leukemic stem cell formation. These mechanisms may also pertain to HSC aging (Arif*, Qiu* al., Cell Stem Cell, 2025; Ghaffari, Cell Stem Cell, 2021). By decelerating the aging process of blood stem cells, age-related blood disorders may be delayed, or perhaps – in some cases – prevented. Identifications of these mechanisms is likely to lead to potential strategies for restoring youthful vitality in the aging body.
Our studies on lysosomal and mitochondrial regulation of hematopoietic stem and progenitor cells build on our initial discovery that the longevity-associated FOXO transcription factors – specifically non redundant functions of FOXO3 in HSCs – are key regulators of stem cell activity, followed by more than a decade of work defining their functions (Yalcin et al., JBC, 2008; EMBOJ, 2010; Zhang et al., Nature Cell Biology, 2011; Rimmelé et al., Stem Cell Reports, 2014; EMBO Reports, 2015; Liang et al., Cell Cycle, 2016; Bigarella et al., JBC, 2017).
Leukemic Stem Cells and Their Organelles
Leukemic stem cells (LSCs) can resist therapy and reestablish leukemia even years after a patient achieves remission. Understanding the biology of these cells may open the door to new and more effective treatments. Our work suggests that pathways normally responsible for protecting healthy stem cells from age-related cellular damage can become dysregulated with aging and intersect with pathways that drive leukemia. We have found that mitochondrial remodeling plays a central role in this process (Bigarella, Liang et al., in revision).
By leveraging unique mitochondrial features, we have identified potent human HSCs (Qiu et al., Blood Advances, 2021) and therapy-resistant LSCs in a myeloproliferative neoplasm (MPN) model (Qiu & Ghaffari, in preparation).
Erythroid Cell Enucleation, Production and Mitochondria
Erythropoiesis is the process of making red blood cells (RBCs) from hematopoietic stem and progenitor cells. Anemia or reduced capacity of RBCs to carry oxygen constitutes a major health problem associated with many disorders and with aging. In response to loss or reduced production of RBCs, blood-forming stem and progenitor cells are activated to produce RBCs. RBCs lack nucleus. Enucleation is a limiting step in erythroid cell production ex vivo.
Ghaffari lab identified FOXO3 as one of the main transcription factors that coordinates the physiological maturation process of erythroid cells and important for erythroid cell enucleation (Marinkovic et al., JCI, 2007; Yalcin et al., EMBOJ, 2010; Zhang et al., AJH 2014; Liang et al., PLoS Genetics, 2015). The positioning of mitochondria behind the nucleus was shown to be essential for erythroid enucleation (Liang et al., Blood Advances, 2021) and FOXO3 was found to be critical for both mitochondrial function and enucleation in late erythroid precursors (Liang et al., PLoS Genetics, 2015). Additional work found that FOXO3 and p21 mediate erythroid apoptosis in beta-thalassemia (Liang, et al., Blood Advances, 2023). Currently the lab is focused on identifying specific mitochondrial functions that control erythroid cell production (Menon and Ghaffari, Exp. Hem, 2021; Liang et al. Blood Adv., 2021; Menon et al., British Journal of Hematology, 2024). Our goal is to contribute to advancing the knowledge necessary for improving the therapy outcome in erythroid disorders.
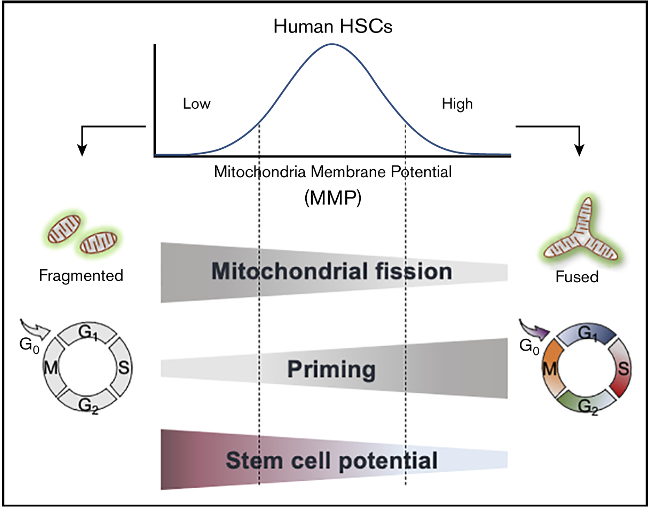
Low mitochondrial membrane potential (MMP) enriches for highly potent human HSCs. Qiu et al., Blood Advances 2021.