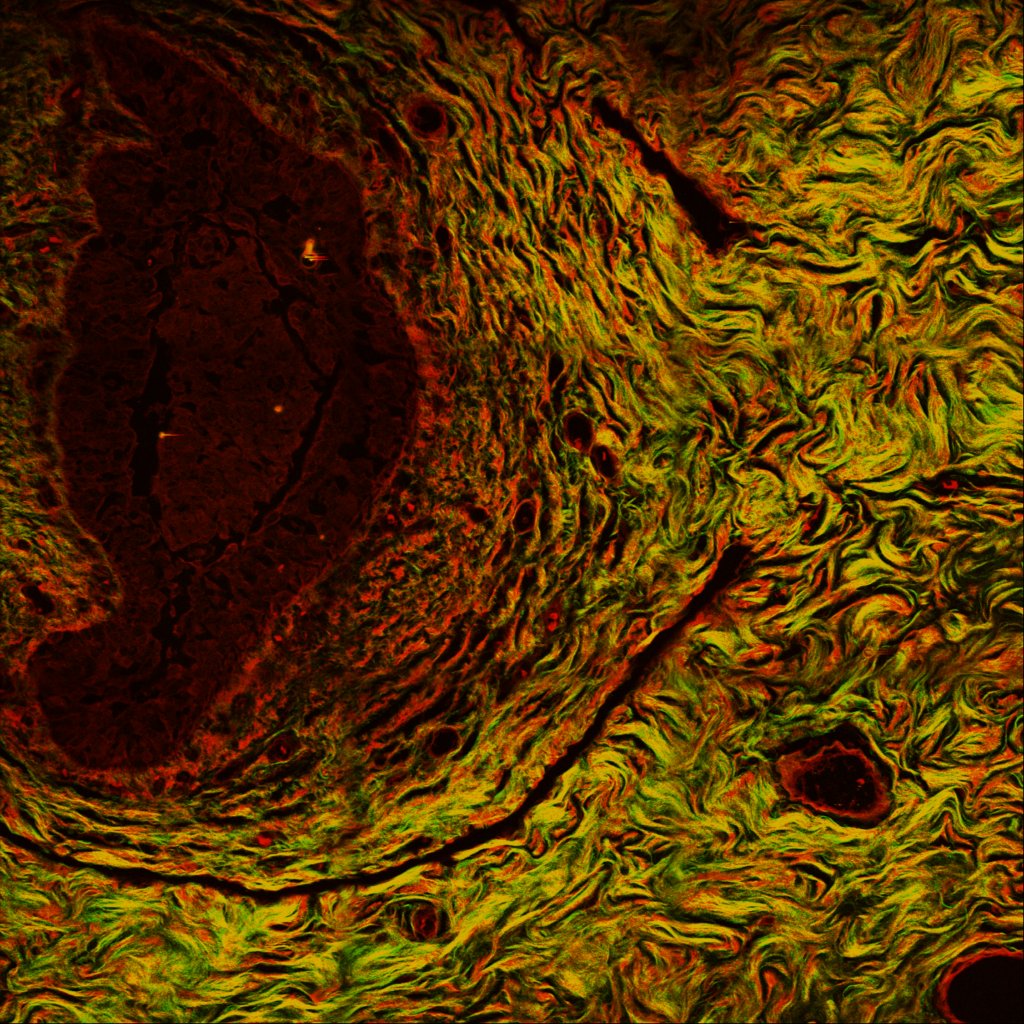
Second harmonic generation (SHG) image on a breast cancer section. Green: collagen; Red: nonspecific tissue
Our laboratory focuses on breast cancer. One area of interest that we are currently pursuing is the link between pregnancy and breast cancer. Pregnancy at a young age (before the age of 25) is associated with a reduction in the overall life-time risk of developing breast cancer. This observation represents the protective effect of pregnancy. However, pregnancy is also associated with a transient increase in risk of breast cancer in all women, which peaks at 6 years after pregnancy. Further, the risk increases with the age of the mother at first pregnancy. Since women in developed countries tend to have children after the age of 30, pregnancy represents an important etiological factor in breast cancer today.
The current definition of pregnancy-associated breast cancer (PABC) is empirically limited to breast cancers arising within 2 years of pregnancy. The 15-year survival of women diagnosed with breast cancer 1 and 2 years after giving birth is 38% and 51%, respectively, compared to 65% in age-matched nulliparous women, suggesting the aggressive nature of the disease. The restricted time frame of diagnosis allowed by the current definition fuels the notion that PABC is a rare phenomenon. However, since the epidemiological data demonstrate that the increase in risk peaks 6 years following pregnancy, PABC arising beyond the 1 to 2 year window may represent the vast majority of cases. If so, PABC may represent a considerable fraction of breast cancers.
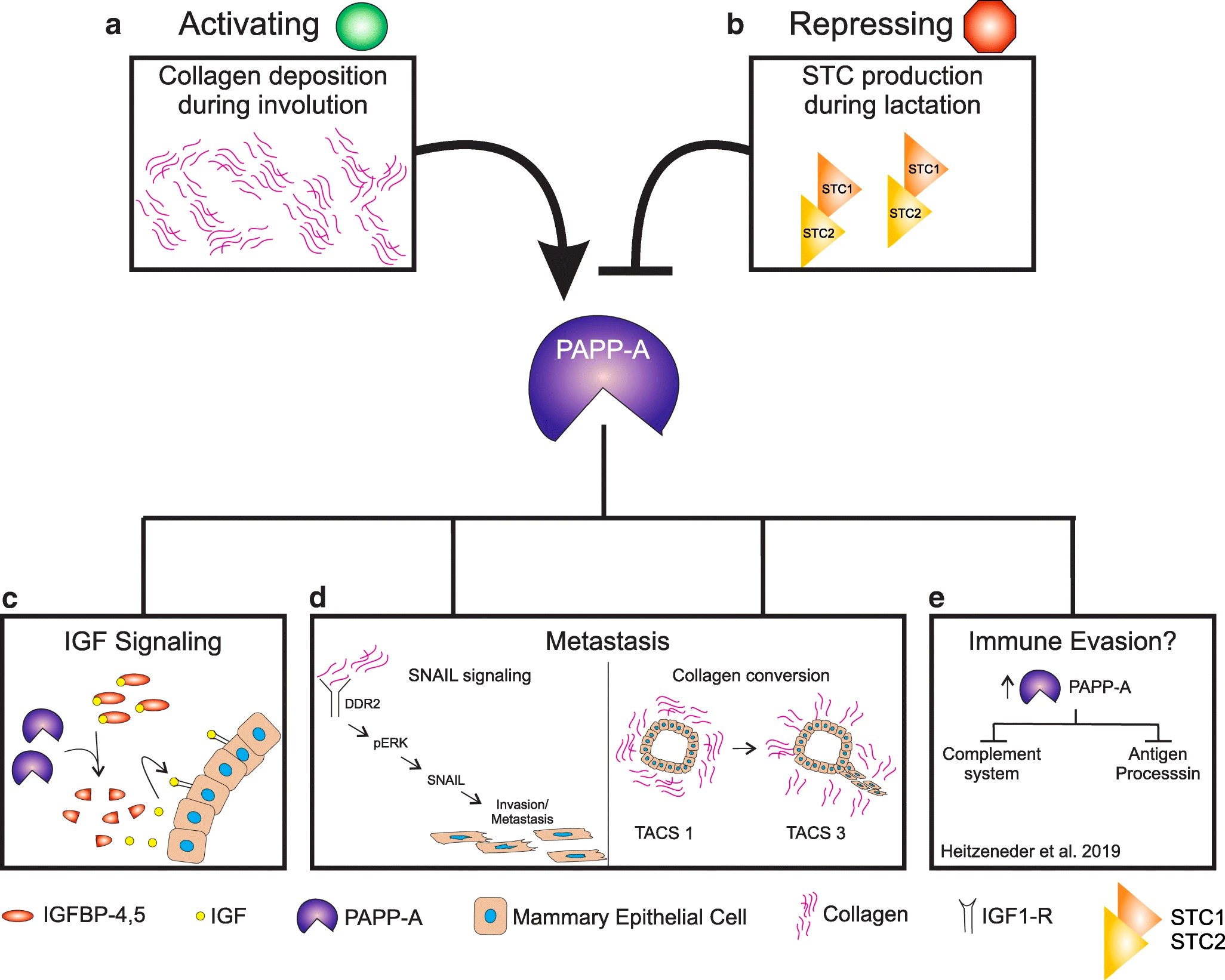
One major breakthrough regarding PABC is the discovery that involution of the breast following pregnancy is tumorigenic. Work in our laboratory has identified the protease Pappalysin-1 (PAPP-A) as a involution-dependent oncogene as it is activated specifically during involution. PAPP-A cleaves the inhibitors of IGF signalling IGFBP-4 and 5. We found that expression of PAPP-A increases the deposition of collagen during involution and that collagen enhances the ability of PAPP-A to cleave its substrates therefore establishing a positive feedback loop. We also found that lactation opposes the oncogenic function of PAPP-A.
This observation is in line with a large analysis of the effect of breastfeeding using combined results from 47 studies, involving a total of 50,302 women, revealed that extended lactation is protective against breast cancer. This study suggested that the cumulative risk of breast cancer could be reduced by half should the period of breastfeeding be increased. In addition, breastfeeding was found to be protective against more aggressive tumors, but the mediators of the protective effect of lactation have not been identified.
In the future, we aim at further understanding the role of breastfeeding on the oncogenic function of PAPP-A–driven PABC. Further, we are interested in exploring the possibility that altered collagen in an aging breast may favor the oncogenic function of PAPP-A.