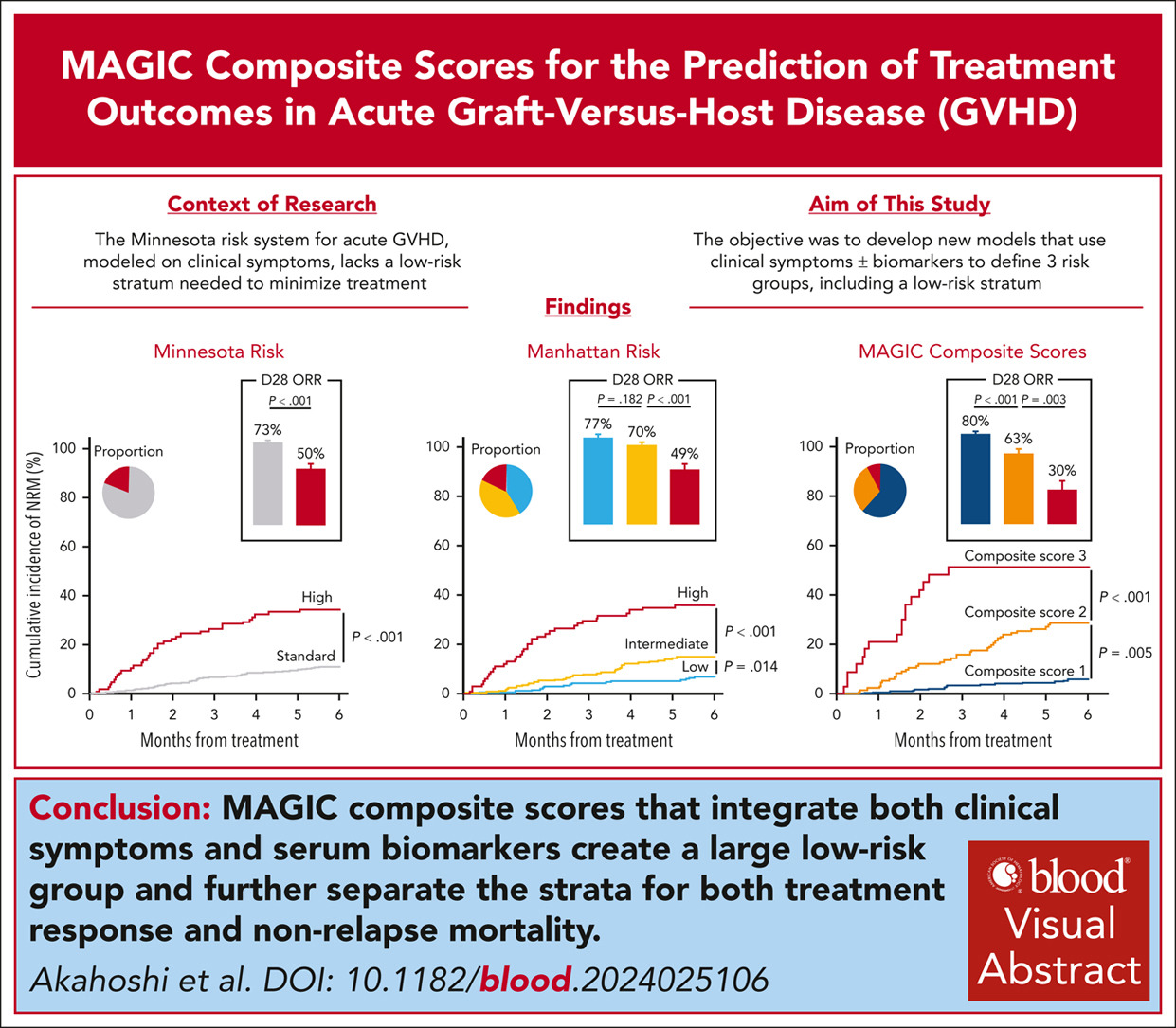
Novel MAGIC composite scores using both clinical symptoms and biomarkers best predict treatment outcomes of acute GVHD
Blood, 2024; 144:1010-1021
Acute graft-versus-host disease (GVHD) grading systems that use only clinical symptoms at treatment initiation such as the Minnesota risk identify standard and high-risk categories but lack a low-risk category suitable to minimize immunosuppressive strategies. We developed a new grading system that includes a low-risk stratum based on clinical symptoms alone and determined whether the incorporation of biomarkers would improve the model’s prognostic accuracy. We randomly divided 1863 patients in the Mount Sinai Acute GVHD International Consortium (MAGIC) who were treated for GVHD into training and validation cohorts. Patients in the training cohort were divided into 14 groups based on similarity of clinical symptoms and similar nonrelapse mortality (NRM); we used a classification and regression tree (CART) algorithm to create three Manhattan risk groups that produced a significantly higher area under the receiver operating characteristic curve (AUC) for 6-month NRM than the Minnesota risk classification (0.69 vs 0.64, P = .009) in the validation cohort. We integrated serum GVHD biomarker scores with Manhattan risk using patients with available serum samples and again used a CART algorithm to establish 3 MAGIC composite scores that significantly improved prediction of NRM compared to Manhattan risk (AUC, 0.76 vs 0.70, P = .010). Each increase in MAGIC composite score also corresponded to a significant decrease in day 28 treatment response (80% vs 63% vs 30%, P < .001). We conclude that the MAGIC composite score more accurately predicts response to therapy and long-term outcomes than systems based on clinical symptoms alone and may help guide clinical decisions and trial design.
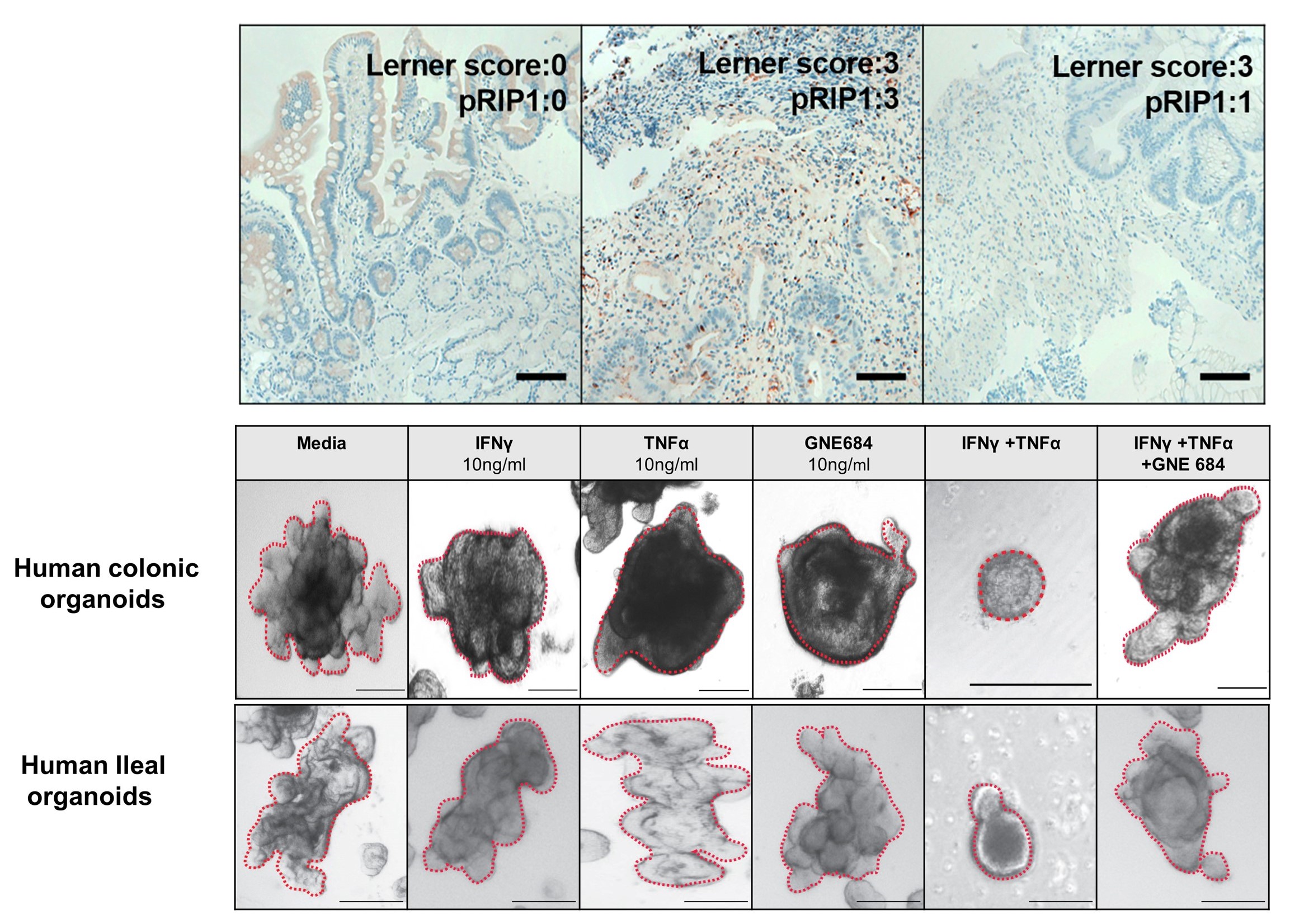
Inhibition of RIP1 improves immune reconstitution and reduces GVHD mortality while preserving graft-versus-leukemia effects
Sci. Transl. Med. 2023 Dec 20;15(727)
Graft-versus-host disease (GVHD) remains the major cause of morbidity and nonrelapse mortality (NRM) after hematopoietic cell transplantation (HCT). Inflammatory cytokines mediate damage to key GVHD targets such as intestinal stem cells (ISCs) and also activate receptor interacting protein kinase 1 (RIP1; RIPK1), a critical regulator of apoptosis and necroptosis. We therefore investigated the role of RIP1 in acute GVHD using samples from HCT patients, modeling GVHD damage in vitro with both human and mouse gastrointestinal (GI) organoids, and blocking RIP1 activation in vivo using several well-characterized mouse HCT models. Increased phospho-RIP1 expression in GI biopsies from patients with acute GVHD correlated with tissue damage and predicted NRM. Both the genetic inactivation of RIP1 and the RIP1 inhibitor GNE684 prevented GVHD-induced apoptosis of ISCs in vivo and in vitro. Daily administration of GNE684 for 14 days reduced inflammatory infiltrates in three GVHD target organs (intestine, liver, and spleen) in mice. Unexpectedly, GNE684 administration also reversed the marked loss of regulatory T cells in the intestines and liver during GVHD and reduced splenic T cell exhaustion, thus improving immune reconstitution. Pharmacological and genetic inhibition of RIP1 improved long-term survival without compromising the graft-versus-leukemia (GVL) effect in lymphocytic and myeloid leukemia mouse models. Thus, RIP1inhibition may represent a nonimmunosuppressive treatment for GVHD.
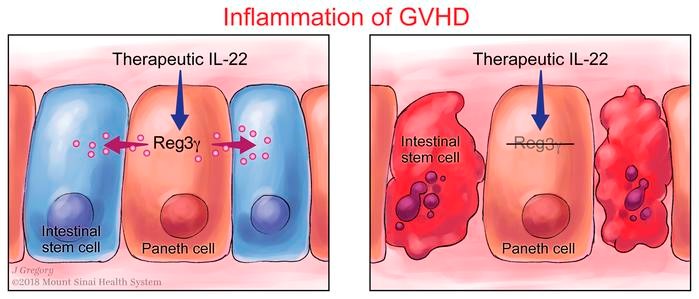
Survival signal REG3α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease
Journal of Clinical Investigation, 2018; 128:4970-79
Graft-versus-host disease (GVHD) in the gastrointestinal (GI) tract remains the major cause of morbidity and nonrelapse mortality after BM transplantation (BMT). The Paneth cell protein regenerating islet-derived 3α (REG3α) is a biomarker specific for GI GVHD. REG3α serum levels rose in the systematic circulation as GVHD progressively destroyed Paneth cells and reduced GI epithelial barrier function. Paradoxically, GVHD suppressed intestinal REG3γ (the mouse homolog of human REG3α), and the absence of REG3γ in BMT recipients intensified GVHD but did not change the composition of the microbiome. IL-22 administration restored REG3γ production and prevented apoptosis of both intestinal stem cells (ISCs) and Paneth cells, but this protection was completely abrogated in Reg3g−/− mice. In vitro, addition of REG3α reduced the apoptosis of colonic cell lines. Strategies that increase intestinal REG3α/γ to promote crypt regeneration may offer a novel, nonimmunosuppressive approach for GVHD and perhaps for other diseases involving the ISC niche, such as inflammatory bowel disease.
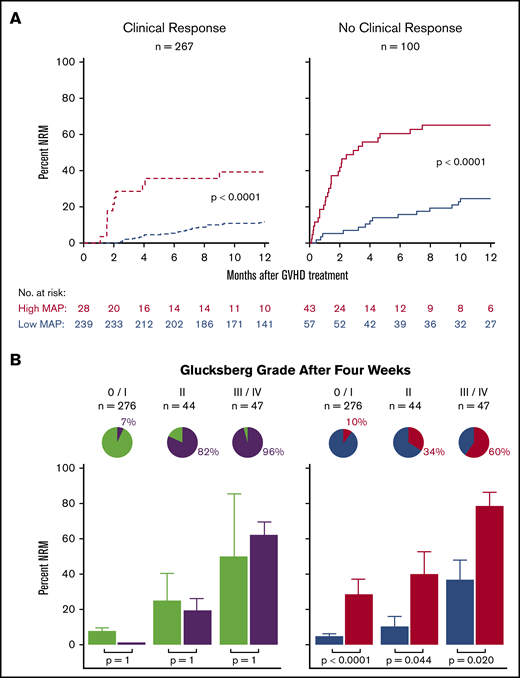
The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease
Blood Advances, 2019; 3:4034-4042
The Mount Sinai Acute GVHD International Consortium (MAGIC) algorithm probability (MAP), derived from 2 serum biomarkers, measures damage to crypts in the gastrointestinal tract during graft-versus-host disease (GVHD). We hypothesized that changes in MAP after treatment could validate it as a response biomarker. We prospectively collected serum samples and clinical stages of acute GVHD from 615 patients receiving hematopoietic cell transplantation in 20 centers at initiation of first-line systemic treatment and 4 weeks later. We computed MAPs and clinical responses and compared their abilities to predict 6-month nonrelapse mortality (NRM) in the validation cohort (n = 367). After 4 weeks of treatment, MAPs predicted NRM better than the change in clinical symptoms in all patients and identified 2 groups with significantly different NRM in both clinical responders (40% vs 12%, P < .0001) and nonresponders (65% vs 25%, P < .0001). MAPs successfully reclassified patients for NRM risk within every clinical grade of acute GVHD after 4 weeks of treatment. At the beginning of treatment, patients with a low MAP that rose above the threshold of 0.290 after 4 weeks of treatment had a significant increase in NRM, whereas patients with a high MAP at onset that fell below that threshold after treatment had a striking decrease in NRM that translated into clear differences in overall survival. We conclude that a MAP measured before and after treatment of acute GVHD is a response biomarker that predicts long-term outcomes more accurately than change in clinical symptoms. MAPs have the potential to guide therapy for acute GVHD and may function as a useful end point in clinical trials.

Effective treatment of low-risk acute GVHD with itacitinib monotherapy
Blood, 2023; 141:481-489
The standard primary treatment for acute graft-versus-host disease (GVHD) requires prolonged, high-dose systemic corticosteroids (SCSs) that delay reconstitution of the immune system. We used validated clinical and biomarker staging criteria to identify a group of patients with low-risk (LR) GVHD that is very likely to respond to SCS. We hypothesized that itacitinib, a selective JAK1 inhibitor, would effectively treat LR GVHD without SCS. We treated 70 patients with LR GVHD in a multicenter, phase 2 trial (NCT03846479) with 28 days of itacitinib 200 mg/d (responders could receive a second 28-day cycle), and we compared their outcomes to those of 140 contemporaneous, matched control patients treated with SCSs. More patients responded to itacitinib within 7 days (81% vs 66%, P = .02), and response rates at day 28 were very high for both groups (89% vs 86%, P = .67), with few symptomatic flares (11% vs 12%, P = .88). Fewer itacitinib-treated patients developed a serious infection within 90 days (27% vs 42%, P = .04) due to fewer viral and fungal infections. Grade ≥3 cytopenias were similar between groups except for less severe leukopenia with itacitinib (16% vs 31%, P = .02). No other grade ≥3 adverse events occurred in >10% of itacitinib-treated patients. There were no significant differences between groups at 1 year for nonrelapse mortality (4% vs 11%, P = .21), relapse (18% vs 21%, P = .64), chronic GVHD (28% vs 33%, P = .33), or survival (88% vs 80%, P = .11). Itacitinib monotherapy seems to be a safe and effective alternative to SCS treatment for LR GVHD and deserves further investigation.
See More Publications...
2018
Zhao D, Kim Y-H, Jeong S, Greenson JK, Chaudhry MS, Hoepting M, Anderson ER, van den Brink MRM, Peled JU, Gomes ALC, Slingerland AE, Donovan MJ, Harris AC, Levine, JE, Ozbek U, Hooper LV, Stappenbeck TS, Ver Heual AM, Liu T-C, Reddy P, Ferrara JLM. Survival signal REG3-α prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. The Journal of Clinical Investigation. 2018 Aug 14.
Holtan SG, DeFor TE, Panoskaltsis-Mortari A, Khera N, Levine JE, Flowers ME, Lee SJ, Inamoto Y, Chen GL, Mayer S, Arora M, Palmer J, Cutler CS, Arai S, Lazaryan A, Newell LF, Jagasia MH, Pusic I, Wood WA, Renteria AS, Yanik, G, Hogan WJ, Hexner E, Holler E, Bunworasate U, Efebera YA, Ferrara JL, Pidala J, Howard A, Wu, Bolaños-MeadeJ, HV, Alousi A, Blazar BR, Weisdorf DJ, & MacMillan ML. Amphiregulin modifies the Minnesota Acute Graft-versus-Host Disease Risk Score: results from BMT CTN 0302/0802. Blood Advances. 2018. 2(15), 1882-1888.
Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, Efebera YA, Hogan WJ, Wölfl M, Qayed M, Hexner EO, Wudhikarn K, Ordemann R, Young R, Shah J, Hartwell MJ, Chaudhry M, Aziz M, Etra A, Yanik GA, Kröger N, Weber D, Chen YB, Nakamura R, Rösler W, Kitko CL, Harris AC, Pulsipher M, Reshef R, Kowalyk S, Morales G, Torres I, Özbek U, Ferrara JLM, Levine JE. MAGIC biomarkers predict long term outcomes for steroid-resistant acute GVHD. Blood, 2018 Mar 15
2017
Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, Aziz M, Hogan WJ, Ayuk F, Efebera YA, Hexner EO, Bunworasate U, Qayed M, Ordemann R, Wölfl M, Mielke S, Pawarode A, Chen YB, Devine S, Harris AC, Jagasia M, Kitko CL, Litzow MR, Kröger N, Locatelli F, Morales G, Nakamura R, Reshef R, Rösler W, Weber D, Wudhikarn K, Yanik GA, Levine JE, Ferrara JL. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017 Feb 9;2(3):e89798.
2016
Renteria AS, Levine JE, Ferrara JL. Development of a biomarker scoring system for use in graft-versus-host disease. Biomark Med. 2016 Aug;10(8):793-5.
2015
Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, Magenau J, Weisdorf DJ, Ho VT, Bolaños-Meade J, Alousi AM, Ferrara JL. A Prognostic Score for Acute Graft-Versus-Host Disease Based On Biomarkers: A Multicenter Study. The Lancet Haematology, Elsevier LTD. 2015 Jan, 2:e21-29.
Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, Koehn BH, Pfeifer D, Taylor PA, Prinz G, Dierbach H, Stickel N, Beck Y, Warncke M, Junt T, Schmitt-Graeff A, Nakae S, Follo M, Wertheimer T, Schwab L, Devlin J, Watkins SC, Duyster J, Ferrara JL, Turnquist HR, Zeiser R, Blazar BR. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood. 2015 May 14;125(20):3183-92.
