COVID-19
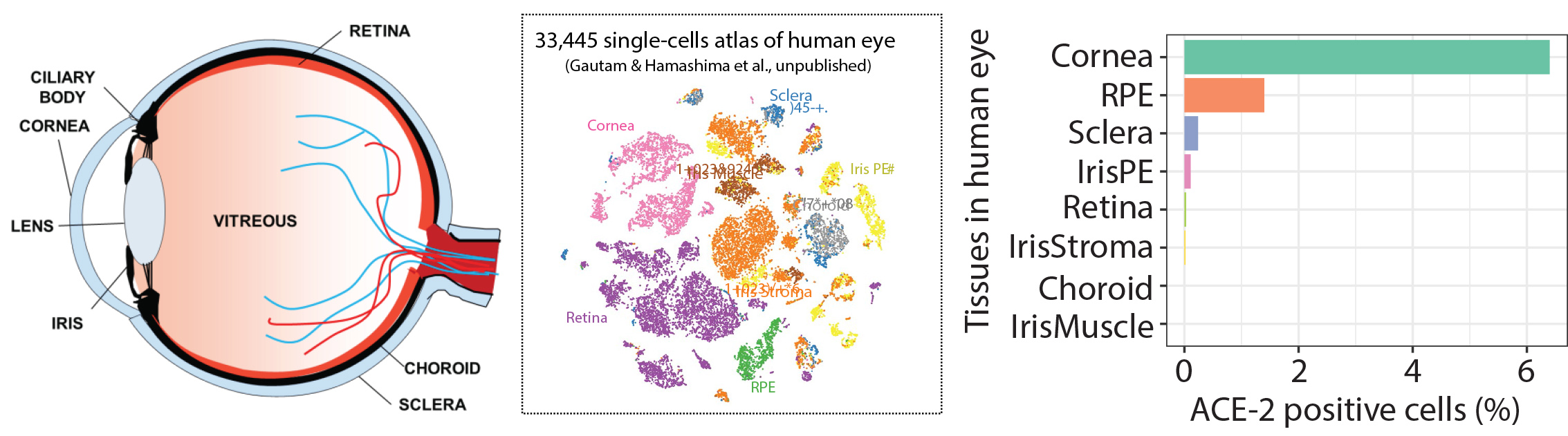
With the recent outbreak of SARS-CoV-2 we have focused on understanding the risk of infection through the eye. Using generous donations we studied the gene expression of adult human eye tissues for the expression of receptors hypothesized to be targeted for cellular internalization into human cells ACE-2 and TMPRSS2. We find that the Cornea possesses the highest percentage of cell which express the receptors. Current studies are evaluating infectivity and potential compounds able to prevent infection for the development of anti-SARS-CoV-2 eye drops.
Retinal regeneration
The neural retina in early vertebrates has demonstrated surprising flexibility in repairing damaged tissue. The human retina, while seemingly less regenerative has also shown some plasticity and ability to repair. Our approach in exploring the regenerative potential of the human eye is to study the eyes of generous organ donors. We have developed methods where we can keep eye tissues in culture while maintaining their normal physiology. We can also expand the cells so that we have sufficient quantities to study the eye in new ways never before possible. We examine their epigenetic states, electrophysiology, and morphology from normal and diseased donors. From this we are drawing connections between their cellular physiology and epigenetic landscape with the hope of gaining insight on the changes we may control to restore lost function. Moreover we are using these cultures as sources for cell replacement therapies for eye disease.
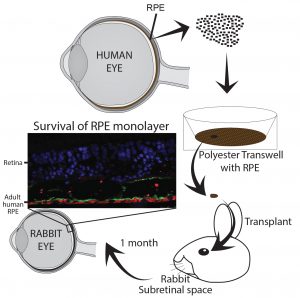
Using adult human RPE as a source for
cell replacement therapy for treating Age-related Macular Degeneration
Age-related macular Degeneration (AMD) is an age related disease where in the U.S 15% over the age of 50 years is diagnosed. Early symptoms of the disease is marked by a deposition of misfolded proteins behind the retina and loss of retinal pigment epithelium a cell layer essential for phototransduction and vision. Because of this, one exciting strategy for these patients is to replace diseased RPE with healthy RPE in the hope they will restore normal function and prevent disease progression and vision loss. Adult human RPE (ahRPE) received from generous organ donors are an exciting source for cell replacement therapies. ahRPE have mature native physiology, and may aid in functional integration. Moreover, ahRPE have stable MHC expression allowing donor/host matching and utilizing long-established organ transplantation donor host matching protocols. Using tissue from adult human donors is less ethically controversial increasing the likelihood of acceptance and adoption. Lastly, adult cadaver donor-derived tissue is an inexhaustible supply, and based on our current culture protocols, we can obtain enough pure and functional RPE cells from each donor to treat up to 10,000 patients.
As a member of the Retinal Stem Cell Consortium (NYSTEM) that will be filing an IND with the FDA in 2017 for the use of adult human RPE for subretinal transplantation as a suspension for the treatment of AMD, we have contributed to the development of a GMP production pipeline from donor eye dissection to final cell product, including the establishment of release tests, quality control assays and surgical implantation protocols. Pre-clinical studies are now needed to evaluate the promise of ahRPE transplanted as a monolayer for patients with dry AMD.
There is still much work to do and we are working to established improved methods for improving functional integration and evasion from the immune system.
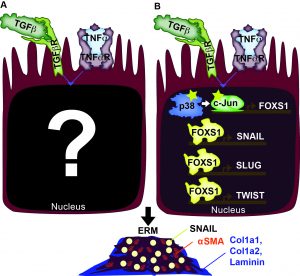
Cellular and Molecular foundation of Epiretinal Membrane Formation
Epiretinal membranes (ERMs) are a form of metaplasia in the vitreous often found in patients with inflammation-associated eye diseases, including retinal detachment, proliferative diabetic retinopathy, and uveitis. Both TGFb and TNFa activate ERM formation. Upon retinal tear, cells of the retina, particularly retinal glia (RG) and retinal pigment epithelium (RPE), are exposed to abnormally high levels of TGFb and TNFa. Elevated levels of TGFb and TNFa have been implicated in metaplasias of many other tissues, including cancers and neoplasias. With our present understanding of TGFb and TNFa, we are at a crossroads where we need to examine their downstream effects, both transcriptionally and epigenetically, to gain a clearer picture of their synergistic impact. We have identified three downstream effectors of the synergistic effect of TGFb and TNFa, which includes p38 MAPK, c-Jun, and FOXS1. We are exploring how these factors and others lead to the reprogramming of the cell state epigenetically which results in pathological states.