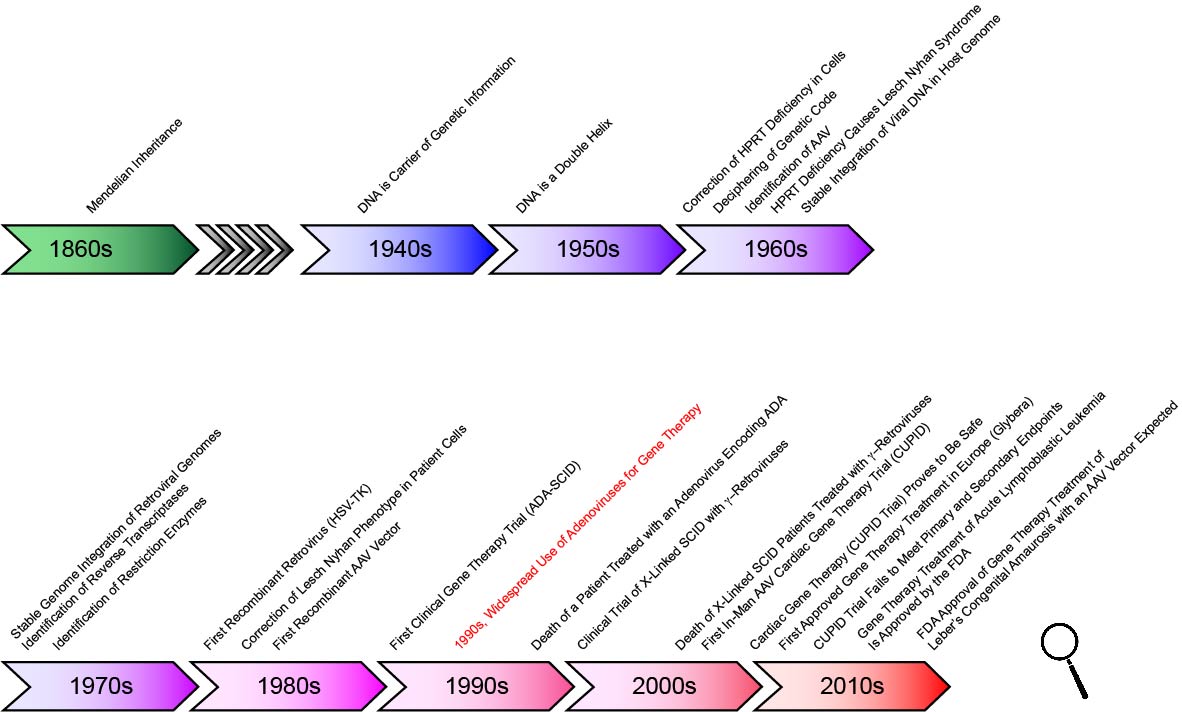
In the nineteenth century, Gregor Mendel conducted experiments that revealed the essential laws of heredity, making it clear that important features are inherited by a defined and predictable mechanism. The carrier of this information remained unknown, however, until the 1940s, when Oswald Avery and colleagues at Rockefeller University in New York identified the carrier of genetic information, DNA. In the early 1950’s, James Watson, Francis Crick, Rosalind Franklin and Maurice Wilkins determined that DNA is a double helix — and they hypothesized that this structure could be used to replicate and pass on genetic information. Their work was followed by the ingenious experiments of Marshall W. Nirenberg and Har Gobind Khorana in the 1960s that resulted in the deciphering of the genetic code.
In the 1970s, the discovery of restriction enzymes and their use in molecular biology — by Werner Arber, Daniel Nathans, and Hamilton O. Smith — was a watershed moment that fundamentally changed life sciences forever. This discovery made genetic manipulation possible — first in bacteria, and later in all species. In fact only 11 years later, in 1981, the first transgenic mice were created. It is remarkable that in 1989, less than 20 years after the discovery of the first restriction enzyme, the first gene therapy experiment to treat melanoma was approved by the NIH, followed in 1990 with the treatment of a four-year-old girl who had a rare immune system disorder.
Recent Past, Present and Future of Gene Therapy
With the decoding of the human genome, there is no shortage in targets for gene therapeutic applications. They range from treatment of classical genetic disorders, often of monogeneic origin, such as cystic fibrosis, lysosomal storage diseases, bleeding disorders, and sickle cell anemia, to more complex disorders with genetic components, such as diabetes, cardiovascular disorders, and cancer. For some of the major diseases, gene therapy is one possible treatment, for others, it is likely the most promising approach. Despite the fact that gene therapy is a scientifically sound concept, however, the translation into the clinic has proven to be challenging.
The first reported clinical success was the treatment of children suffering from X-linked SCID (severe combined immunodeficiency). The therapeutic gene in this case was delivered by a retroviral vector. Tragically, it later became clear that, as a result of an oncogenic insertion of the viral genome, three of the approximately ten treated children acquired a leukemia-like disease. This case and the tragic death of Jesse Gelsinger in a clinical trial using adenoviral vectors are forceful illustrations that gene therapy remains a a challenging therapeutic modality that can be associated with significant risks.
Recently, however, the field has made tremendous progress, and there is now no doubt that, in the future, gene therapy will have an important place in treating a large array of diseases. Strikingly, since the first clinical trial roughly 20 years ago, more than 2,200 clinical trials have been initiated (February 2016; www.wiley.com/legacy/wileychi/genmed/clinical/). Maybe most importantly, in 2018 the FDA approved the treatment of Leber’s congenital amaurosis, which causes early childhood blindness, with an adeno-associated vector encoding the missing gene, RPE65, for clinical use—the first FDA authorization of an in vivo gene therapeutic treatment.
At present, one of the major limitations to advance in the field of gene therapy remains the absence of ideal gene delivery vehicles for many applications. Only further progress in vector development will allow gene therapy to fulfill its potential as one of the main new avenues of medical treatment of the twenty-first century.
Among the most promising candidates for efficient therapeutic gene delivery are vectors based on adeno-associated virus (AAV).
The study of AAV and the development of improved AAV vectors are the principle research areas in our laboratory.