Cardiovascular Epitranscriptomics and Exosomes Laboratory
Research Summary
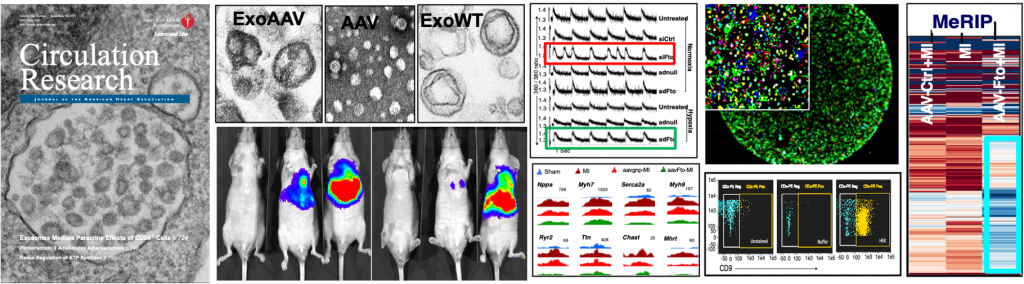
Pre-clinical, translational and basic research in my laboratory investigates exosomes and epitranscriptomic mechanisms in the heart. We were the first to study stem cell-derived bionanovesicles called exosomes and exosomal noncoding RNA-mediated microcommunication for cardiac regeneration. In another first, we are investigating post-transcriptional mRNA methylations (epitranscriptome) in nuclear export and translation of mRNAs and in cardiac regeneration. We use state-of-the-art techniques such as MeRIP sequencing, flow cytometry, electron, super-resolution and confocal microscopy, SILAC-LC-MS, in vitro and in vivo imaging, systems genetics, bionanotechnology, small and large animal models of heart failure to address scientific questions in an innovative way
Exosomes in Cardiac Repair
Pre-clinical and translational research in my laboratory investigates exosomes-mediated microcommunication between stem cells, cardiomyocytes and endothelial cells under physiological and pathological milieu of the heart. Exosomes are naturally secreted nanovesicles that can shuttle fortified or diseased material from the cell of their origin to cells at the vicinity or at a distance. Recently, we have discovered that human stem cells secrete exosomes to mediate repair of a diseased heart by transferring their unique repertoire of miRNAs improving cardiac function and reducing fibrosis. Cell-free exosomes are a key biologically active component of stem cells, which has the potential to replace cell-based therapies with the potential to address their limitations. Further, the cell-free exosomes can be used as vectors for delivering miRNAs, genes, and even viruses and can be a suitable replacement to cellular and gene therapies for cardiac repair and regeneration. We aim to harness the regenerative and communicative potential of stem cell-derived exosomes for novel therapeutic approaches. Our long-term goal is to artificially engineer the powerful nanovesicles for an off-the-shelf-product to treat patients with cardiovascular diseases.
Cardiac Epitranscriptome in Cardiac Remodeling and Regeneration
As epitranscriptomic mechanisms are unraveling, our knowledge about its biological function, especially in organs and tissues and under pathological conditions are limited. Our lab is investigating the biological role of cardiac m6A epitranscriptome and function of an m6A eraser, FTO, in cardiac homeostasis, remodeling and regeneration. Our research provides first evidence that by targeting RNA modifiers, global protein expression can be revived; which may provide new therapeutic opportunities not only for cardiac diseases, but also for a plethora of other diseases, which otherwise cannot be effectively treated by conventional methods.