Gliomas are the most frequently occurring primary brain tumor in children and adults. The most aggressive glioma subtype is the glioblastoma that remains amongst the deadliest form of human cancer. Glioblastomas are universally fatal, with a median survival of just over a year despite the most aggressive treatment regimens.
Recent advances in our understanding of glioma biology have elucidated a complex tumor microenvironment consisting of both neoplastic and non-neoplastic cells. Over the past several years, work from several laboratories, including our independent research laboratory, has revealed that glioma growth is dependent upon growth regulatory signals that emanate from the tumor microenvironment. In this regard, it is essential to recognize that brain tumors are complex network in which communication between neoplastic and non-neoplastic cells drive glioma formation, progression, and therapy resistance.
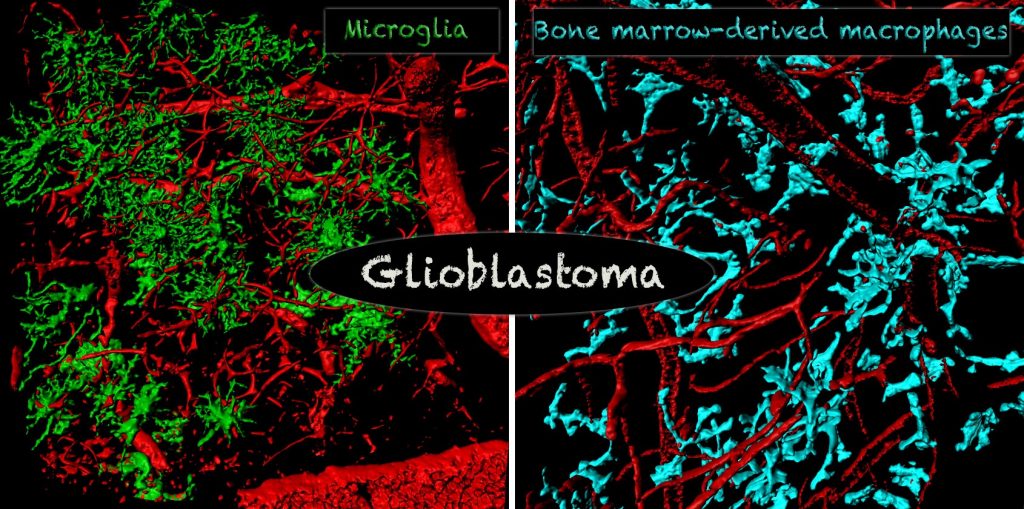
The most abundant non-neoplastic cell population in the glioblastoma microenvironment are tumor-associated macrophages (TAMs). TAMs are recruited to the glioblastoma microenvironment, have immunosuppressive functions, and can release a wide array of growth factors and cytokines in response to factors produced by neoplastic cells.
Although TAMs are genetically stable, they change their expression profile in response to glioblastoma. We have shown that the number, composition, and expression profile of TAMs differ significantly between human GBM subtypes, likely as a result of distinct genetic signatures of tumors, raising the possibility of their differential interaction with tumor cells and T-cells. Currently, our laboratory studies interactions between TAMs with tumor cells and T-cells using various genetically engineered mouse models and human patient samples. We are using multiple techniques, including single-cell RNA-seq and CyTOF for human and mouse glioblastoma immune cell profiling.
