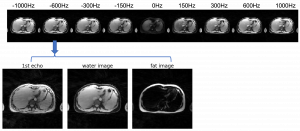
Liver glycogen imaging
The liver is responsible for maintaining blood glucose homeostasis. It is able to deposit large amounts of glucose in the form of glycogen and release glucose rapidly from its glycogen storage when needed. The hepatic glycogen metabolism maybe altered in diseases such as glycogen storage disorders, diabetes mellitus, and liver cirrhosis. Liver biopsy allows direct quantification of glycogen concentration, but it is highly invasive and cannot easily be repeated. 13C nuclear magnetic resonance (NMR) spectroscopy provides a non-invasive way to detect glycogen in the liver, but it is not clinically feasible due to the requirement of specialized imaging hardware. Recently, we have discovered a new contrast mechanism that allows the detection of glycogen based on its nuclear Overhauser enhancement (GlycoNOE). This method enables us to detect glycogen in vivo using standard MRI hardware. In our initial mice study, we found a linear relationship between glycoNOE signal and glycogen concentration and demonstrated that the change in liver glycogen could be measured dynamically. In this project, we aim to develop a new imaging technique to utilize this contrast mechanism in the human liver. To achieve this we will (1) develop and optimize a fast, 3D free-breathing, fat-water separated imaging approach that is suitable for glycoNOE detection in the human liver; (2) compare the NOE signal measured at fed and fasting state to validate that the change in signal reflects the change in glycogen content; and (3) dynamically measure the change of glycogen upon glucagon injection, which further validates the glycoNOE signal and also measures the rate of glycogen degradation. The new imaging technology will be applicable to brain and body imaging based on the nuclear Overhauser enhancement / chemical exchange processes. By providing a non-invasive, real time measure of the change in glycogen level in the liver, it will be possible to study glycogen metabolism in health and in diseases such as diabetes mellitus, glycogen storage diseases, and other liver conditions.
In collaboration with Dr. Li Feng https://med.nyu.edu/faculty/li-feng; https://www.mrfengl.com/bio
 |
 |
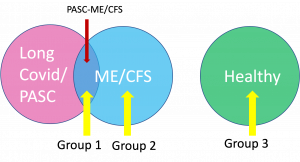
Structural and Metabolic Imaging of Long COVID and Chronic Fatigue Syndrome
A large and increasing number of patients who have been infected with SARS-CoV-2, the virus that causes COVID-19, continue to experience a constellation of symptoms long past the time that they have recovered from the initial illness (long-COVID). The most frequently reported symptoms were fatigue, post exertional malaise and cognitive dysfunction, which are the main symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Clinically, many long-COVID patients fulfill the diagnostic criteria for ME/CFS. An important question is whether those with COVID ME/CFS are essentially the same as those with non-COVID ME/CFS and should be evaluated and treated similarly. If so, this group of COVID-related patients’ CFS could inform us about the features and mechanisms of ME/CFS in general. Although it was suggested that the brain is the organ responsible for both forms of ME/CFS, currently no specific neuroimaging biomarkers have been identified. In this project, we aim to conduct comprehensive neurological magnetic resonance imaging (MRI) to compare the similarities and differences between long-COVID and classic ME/CFS patients, as well as to individuals not affected by ME/CFS, in terms of brain anatomical and metabolic features. Another research focus of this project is to assess changes in oxygen metabolism of ME/CFS in vivo using MRI based methods. We will be measuring the oxygen level in the venous blood of sagittal sinus and the global cerebral blood flow using advanced MRI techniques. Using these parameters, we can calculate the global cerebral metabolic rate of oxygen in the brain which can inform us on whether energy failure is present in individuals with ME/CFS and long-COVID, as previously postulated. Finally, the structural volumetric and metabolic parameters measured by MRI will be correlated with patients’ self-reported symptom burden and multidimensional fatigue level.
In collaboration with Dr. Benjamin Natelson https://profiles.mountsinai.org/benjamin-natelson; https://labs.icahn.mssm.edu/natelsonlab/
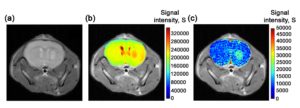
Dynamic Glucose Enhanced MRI
Current clinical MRI contrast agents contain paramagnetic Gadolinium which are not safe for patients with compromised kidney function and a most recent study has shown that these agents leave permanent residuesin brain tissues even in people with normal renal function. There is an urgent need in finding new, biocompatible contrast agents. Glucose is the simplest form of sugar in our everyday life and it is the fuel for all cell activities. Through careful design and optimization, the uptake of glucose can be monitored dynamically using MRI. When injecting glucose into mice implanted with a human brain tumor, very good signal enhancements in the tumor region can be observed. This technic can also be used in studying the perfusion and the permeability properties in human at 7T and 3T during an intravenous glucose tolerance test. We aim to use this contrast mechanism to study the integrity of the blood brain barrier in brain tumors and neurodegenerative diseases such as multiple sclerosis.
 |
||
 |
 |
|
- Xu X, Sehgal AA, Yadav NN, Laterra J, Blair L, Blakeley J, Seidemo A, Coughlin JM, Pomper MG,Knutsson L, van Zijl PCM, d-glucose weighted chemical exchange saturation transfer (glucoCEST)-based dynamic glucoseenhanced (DGE) MRI at 3T: early experience in healthy volunteers and
brain tumor patients.Magn Reson Med. 2020; 84(1):247-262. PMID: 31872916; PMCID: PMC7083699. - Xu X, Xu J, Chan KWY, Liu J, Liu H, Li Y, Chen L, Liu G, van Zijl PCM. GlucoCEST imaging with on-resonance variable delay multiple pulse (onVDMP) MRI. Magn Reson Med. 2019; 81(1): 47-56. PMID: 30058240; PMCID: PMC6258333
- Xu X, Yadav NN, Knutsson L, Hua J, Kalyani R, Hall E, Laterra J, Blakeley J, Strowd R, Pomper M, Barker P, Chan K, Liu G, McMahon MT, Stevens RD, van Zijl PC. Dynamic Glucose-Enhanced (DGE) MRI: Translation to Human Scanning and First Results in Glioma Patients. Tomography. 2015;1(2):105-114. PubMed PMID: 26779568; PubMed Central PMCID: PMC4710854.
- Xu X, Chan KW, Knutsson L, Artemov D, Xu J, Liu G, Kato Y, Lal B, Laterra J, McMahon MT, van Zijl PC. Dynamic glucose enhanced (DGE) MRI for combined imaging of blood-brain barrier break down and increased blood volume in brain cancer. Magn Reson Med. 2015; 74(6):1556-63. PubMed PMID: 26404120; PubMed Central PMCID: PMC4715614.
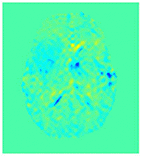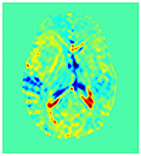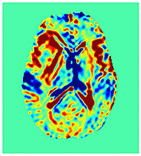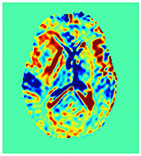
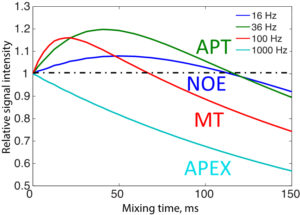
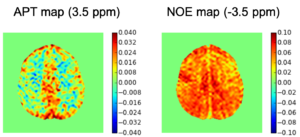
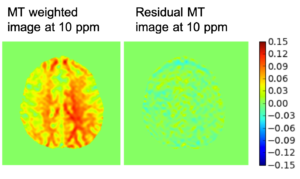
Improving the Sensitivity and Specificity of CEST MRI
Chemical exchange saturation transfer (CEST) imaging is a relatively new MRI technology. It enables a sensitivity enhancement mechanism in which low concentration molecules with exchangeable protons can be detected indirectly through their exchange with water. The enhancement depends on the concentration of the exchangeable protons and the rate of exchange. In vivo, the conventional magnetization transfer contrast which results from the semi-solid compounds is a major confounder of CEST contrast reducing its sensitivity and specificity. I am interested in developing novel MRI method to increase specificity of CEST. My collaborators and I have developed a framework of saturation scheme called variable delay multi pulse (VDMP) CEST. In this method, the inter-pulse delay of a saturation pulse train is varied to act as an exchange rate filter to separate desired CEST effects from background MT contrast and separating the fast-exchanging and slow-exchanging compounds. In addition, the sequence can also be tuned towards imaging fast exchanging proton with high sensitivity. We are always in searching of highly specific and efficient way of generating CEST contrast.
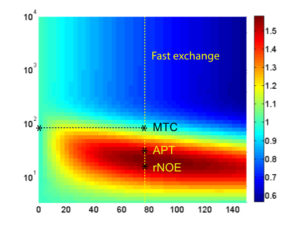 |
 |
 |
 |
||
- Xu X, Yadav NN, Zeng H, Jones CK, Zhou J, van Zijl PC, Xu J. Magnetization transfer contrast-suppressed imaging of amide proton transfer and relayed nuclear overhauser enhancement chemical exchange saturation transfer effects in the human brain at 7T. Magn Reson Med. 2016 Jan;75(1):88-96. PubMed PMID: 26445350; PubMed Central PMCID: PMC4715767.
- Lin Chen, Xiang Xu, Haifeng Zeng, Kannie WY Chan, Nirbhay Yadav, Shuhui Cai, Kathryn J Schunke, Nauder Faraday, Peter CM van Zijl, Jiadi Xu*, Separating fast and slow exchange transfer and magnetization transfer using off‐resonance variable‐delay multiple‐pulse (VDMP) MRI, Magnetic Resonance in Medicine, 80, 1568-1576(2018). PMID: 29405374; PMCID: PMC6077103
- Xu X, Xu J, Chan KWY, Liu J, Liu H, Li Y, Chen L, Liu G, van Zijl PCM. GlucoCEST imaging with on-resonance variable delay multiple pulse (onVDMP) MRI. Magn Reson Med. 2019; 81(1): 47-56. PMID: 30058240; PMCID: PMC6258333
- Xu J, Chan KW, Xu X, Yadav N, Liu G, van Zijl PC. On-resonance variable delay multipulse scheme for imaging of fast-exchanging protons and semisolid macromolecules. Magn Reson Med. 2017 Feb;77(2):730-739. PubMed PMID: 26900759; PubMed Central PMCID: PMC5191984
Ultrafast CEST Spectroscopy and Imaging
CEST spectroscopy and imaging is inherently time consuming due to the need of contrast preparation and performing multiple experiments at different frequency offsets. We are interested in developing methods that can speed up CEST spectroscopy and imaging. Previously we have demonstrated that CEST spectroscopy can be performed in an ultrafast fashion if an encoding gradient field is applied to simultaneously with the saturation pulse. When used with an imaging readout, we have also shown that multiple samples can be screened rapidly, allowing high throughput screen of CEST contrast agents. Furthermore, by interleaving a number of saturation and readout periods within a single repetition time, a series of images with different saturation times can be acquired, enabling quantification of exchange rates using variable saturation time approach in a much-accelerated fashion. Currently, we are exploring different acquisition and reconstruction schemes to accelerate CEST imaging using methods such as golden angle radial sparse parallel MRI.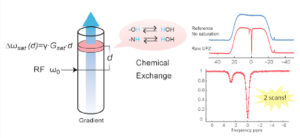
- Xu X, Yadav NN, Song X, McMahon MT, Jerschow A, van Zijl PC, Xu J. Screening CEST contrast agents using ultrafast CEST imaging. J Magn Reson. 2016 Apr;265:224-9. PubMed PMID: 26969814; PubMed Central PMCID: PMC4818714.
- Xu X, Lee JS, Jerschow A. Ultrafast scanning of exchangeable sites by NMR spectroscopy. Angew Chem Int Ed Engl. 2013 Aug 5;52(32):8281-4. PubMed PMID: 23813633; PubMed Central PMCID: PMC3827864.
