Overview
Schizophrenia is a devastating and burdensome illness that afflicts ~1% of the global population. The symptoms of schizophrenia are typically organized into three categories: 1) positive symptoms; 2) negative symptoms; and 3) cognitive symptoms. Positive symptoms consist of hallucinations and delusions, and are together referred to as the psychosis syndrome. Negative symptoms consist of blunted affect, alogia, asociality, avolition and anhedonia. Cognitive symptoms consist of deficits in semantic, explicit and working memory, attention, and executive function. Current pharmacological treatments for schizophrenia are primarily dopamine D2-receptor antagonists that provide limited relief for negative and cognitive symptoms—i.e., they are antipsychotics not “anti-schizophrenia.” Although positive symptoms cause major distress, cognitive symptoms are a hallmark of the disease, affecting most individuals with schizophrenia and being responsible for the greatest reduction in quality of. Importantly, cognitive deficits are among the first symptoms present in at-risk individuals who later develop schizophrenia. This suggests that if we understand their biological mechanisms, we could develop targeted treatments to potentially prevent the development of schizophrenia.
To understand the biological mechanisms of cognitive deficits in schizophrenia the Wengler Lab utilizes a translational and integrative approach. A key focus of the lab is developing neuroimaging measures that can be applied across species (e.g., rodents, non-human primates, humans) and even across measurement methods (e.g., single-unit recordings, calcium imaging, EEG, MRI). We then integrate findings from these measures with biophysical models that are grounded in theoretical neuroscience and allow for the identification of circuit-level alterations that can be exploited for the development of targeted therapeutics. Furthermore, we use findings from both rare (e.g., loss-of-function, CNV) and common (e.g., PRS) genetic variants to provide an integrative understanding of these circuit-level alterations and their potential developmental nature.
A final focus of the Wengler Lab is the development of neuroimaging biomarkers for diagnosing psychiatric illness and predicting treatment response. While conventional approaches have yielded some potential tools, they haven’t been particularly successful for identifying novel therapeutic targets or predicting treatment response. This is partially due to the MRI measures lacking sensitivity for specific biological mechanisms and the poor reliability of some measures. Our view is that for an MRI measure to be clinically useful in these contexts, it must be a mechanistic marker as well as both robust and reliable. The development of such MRI measures includes several steps such as pulse sequence development; the development of advanced analysis methods; optimization of acquisition and analysis methods to improve sensitivity and reliability; benchmarking the reliability so time and money aren’t wasted on measures with low reliability that will hamper clinical utility; and multi-site harmonization methods to allow for the collection of the large-scale datasets required for appropriate development and validation of predictive tools.
Overview
Schizophrenia is a devastating and burdensome illness that afflicts ~1% of the global population. The symptoms of schizophrenia are typically organized into three categories: 1) positive symptoms; 2) negative symptoms; and 3) cognitive symptoms. Positive symptoms consist of hallucinations and delusions, and are together referred to as the psychosis syndrome. Negative symptoms consist of blunted affect, alogia, asociality, avolition and anhedonia. Cognitive symptoms consist of deficits in semantic, explicit and working memory, attention, and executive function. Current pharmacological treatments for schizophrenia are primarily dopamine D2-receptor antagonists that provide limited relief for negative and cognitive symptoms—i.e., they are antipsychotics not “anti-schizophrenia.” Although positive symptoms cause major distress, cognitive symptoms are a hallmark of the disease, affecting most individuals with schizophrenia and being responsible for the greatest reduction in quality of. Importantly, cognitive deficits are among the first symptoms present in at-risk individuals who later develop schizophrenia. This suggests that if we understand their biological mechanisms, we could develop targeted treatments to potentially prevent the development of schizophrenia.
To understand the biological mechanisms of cognitive deficits in schizophrenia the Wengler Lab utilizes a translational and integrative approach. A key focus of the lab is developing neuroimaging measures that can be applied across species (e.g., rodents, non-human primates, humans) and even across measurement methods (e.g., single-unit recordings, calcium imaging, EEG, MRI). We then integrate findings from these measures with biophysical models that are grounded in theoretical neuroscience and allow for the identification of circuit-level alterations that can be exploited for the development of targeted therapeutics. Furthermore, we use findings from both rare (e.g., loss-of-function, CNV) and common (e.g., PRS) genetic variants to provide an integrative understanding of these circuit-level alterations and their potential developmental nature.
A final focus of the Wengler Lab is the development of neuroimaging biomarkers for diagnosing psychiatric illness and predicting treatment response. While conventional approaches have yielded some potential tools, they haven’t been particularly successful for identifying novel therapeutic targets or predicting treatment response. This is partially due to the MRI measures lacking sensitivity for specific biological mechanisms and the poor reliability of some measures. Our view is that for an MRI measure to be clinically useful in these contexts, it must be a mechanistic marker as well as both robust and reliable. The development of such MRI measures includes several steps such as pulse sequence development; the development of advanced analysis methods; optimization of acquisition and analysis methods to improve sensitivity and reliability; benchmarking the reliability so time and money aren’t wasted on measures with low reliability that will hamper clinical utility; and multi-site harmonization methods to allow for the collection of the large-scale datasets required for appropriate development and validation of predictive tools.
Biophysical modeling as a translational bridge for understanding neural ensemble alterations in schizophrenia
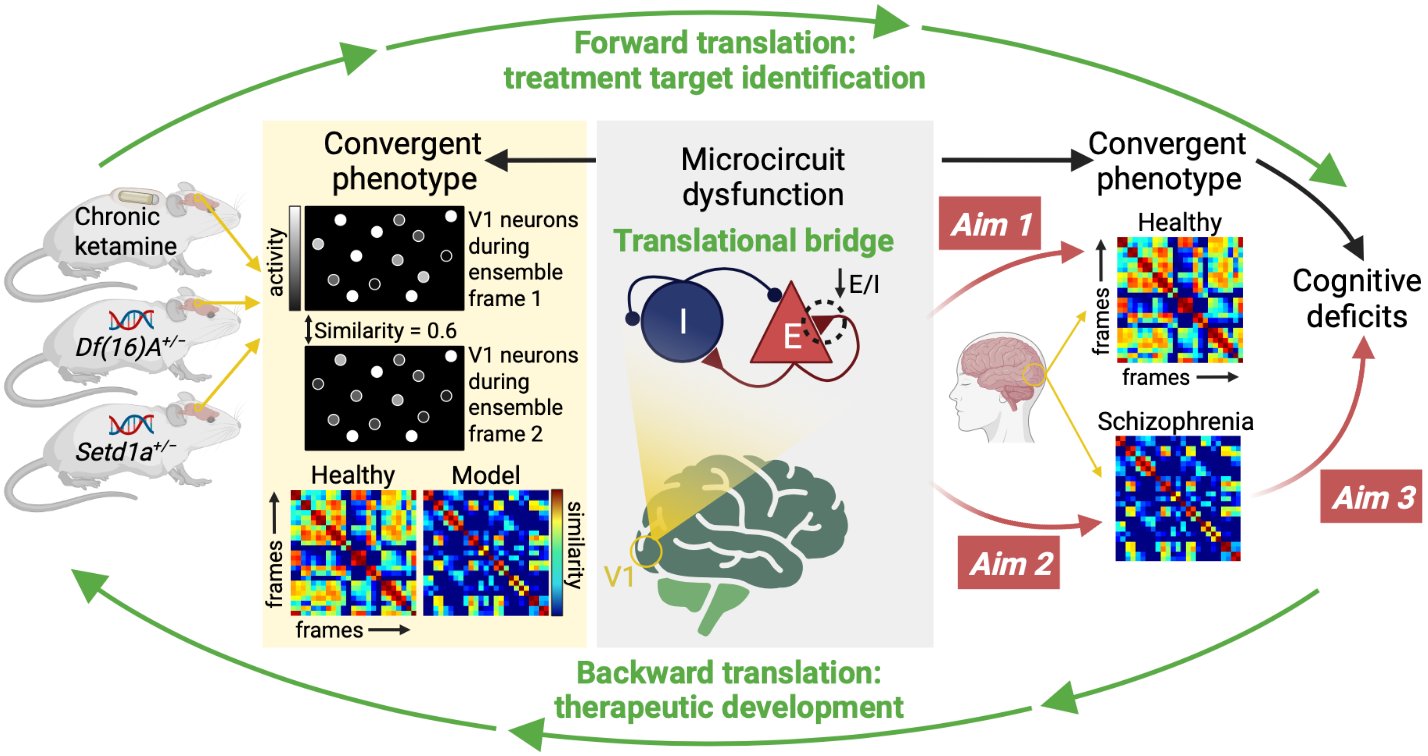
Recent preclinical work has identified reduced neuronal ensemble reliability—similarity in the coactivation patterns across neurons—as a convergent phenotype across three mouse models of schizophrenia-relevant disease processes (Setd1a LoF, 22q11.2 microdeletion CNV, and chronic ketamine). This convergent phenotype provides an entry point for developing novel therapeutics to treat schizophrenia. We have recently developed a biophysical model (a network of excitatory and inhibitory neurons with biologically realistic properties) to recapitulate the reduced ensemble reliability observed in the mouse models and identified a potential biological mechanism of this phenotype. The goal of this work is test for a similar phenotype in patients with schizophrenia using the spatial similarity of fMRI signals and to use the biophysical model to link the findings across species. Finally, because impaired cognition is a convergent behavioral finding across the three mouse models of schizophrenia-relevant disease processes, we aim to link impaired ensemble reliability to cognitive deficits in the patients with schizophrenia.
Biophysical modeling as a translational bridge for understanding neural ensemble alterations in schizophrenia

Recent preclinical work has identified reduced neuronal ensemble reliability—similarity in the coactivation patterns across neurons—as a convergent phenotype across three mouse models of schizophrenia-relevant disease processes (Setd1a LoF, 22q11.2 microdeletion CNV, and chronic ketamine). This convergent phenotype provides an entry point for developing novel therapeutics to treat schizophrenia. We have recently developed a biophysical model (a network of excitatory and inhibitory neurons with biologically realistic properties) to recapitulate the reduced ensemble reliability observed in the mouse models and identified a potential biological mechanism of this phenotype. The goal of this work is test for a similar phenotype in patients with schizophrenia using the spatial similarity of fMRI signals and to use the biophysical model to link the findings across species. Finally, because impaired cognition is a convergent behavioral finding across the three mouse models of schizophrenia-relevant disease processes, we aim to link impaired ensemble reliability to cognitive deficits in the patients with schizophrenia.
An integrative computational interrogation of circuit dysfunction in schizophrenia via neural timescales
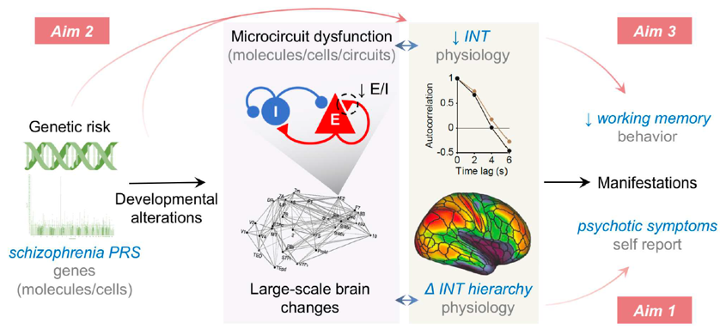
Intrinsic neural timescales (INT) reflect the time window of neural integration within a brain region and have been gaining increasing attention given their relevance to basic neural organizational structure, cognitive function, and neuropsychiatric illness. INT can be easily estimated using resting-state functional MRI (rs-fMRI), such that it can be readily applied to many pre-existing datasets. We recently reported (1) widespread decreases of INT in schizophrenia and (2) localized hierarchical increases of INT linked to psychotic symptoms. The former (1) suggests a trait-like process of widespread cortical E/I reduction driving broad neurocognitive deficits, while the latter (2) suggests a state-dependent compensatory process of circumscribed E/I elevations driving perceptual alterations linked to individual psychotic symptoms. This is consistent with the known relationship between schizophrenia risk genes and the trait-like cognitive deficits that are presumed to be neurodevelopmental in origin. A possible explanation for this set of observations is that polygenic risk for schizophrenia—known to converge on cortical E and I cells—leads to cognitive deficits via a developmental process that results in widespread decrease of recurrent excitation in cortical microcircuits (i.e., reduced E/I ratio) which manifests as widespread INT reduction. To test this hypothesis, this work aims to extend our previous INT findings, evaluate their clinical specificity, their relationships to schizophrenia polygenic risk and developmental changes, and their impact on cognitive function.
An integrative computational interrogation of circuit dysfunction in schizophrenia via neural timescales

Intrinsic neural timescales (INT) reflect the time window of neural integration within a brain region and have been gaining increasing attention given their relevance to basic neural organizational structure, cognitive function, and neuropsychiatric illness. INT can be easily estimated using resting-state functional MRI (rs-fMRI), such that it can be readily applied to many pre-existing datasets. We recently reported (1) widespread decreases of INT in schizophrenia and (2) localized hierarchical increases of INT linked to psychotic symptoms. The former (1) suggests a trait-like process of widespread cortical E/I reduction driving broad neurocognitive deficits, while the latter (2) suggests a state-dependent compensatory process of circumscribed E/I elevations driving perceptual alterations linked to individual psychotic symptoms. This is consistent with the known relationship between schizophrenia risk genes and the trait-like cognitive deficits that are presumed to be neurodevelopmental in origin. A possible explanation for this set of observations is that polygenic risk for schizophrenia—known to converge on cortical E and I cells—leads to cognitive deficits via a developmental process that results in widespread decrease of recurrent excitation in cortical microcircuits (i.e., reduced E/I ratio) which manifests as widespread INT reduction. To test this hypothesis, this work aims to extend our previous INT findings, evaluate their clinical specificity, their relationships to schizophrenia polygenic risk and developmental changes, and their impact on cognitive function.
Neuromelanin-sensitive MRI as a biomarker of catecholamine function in psychiatry
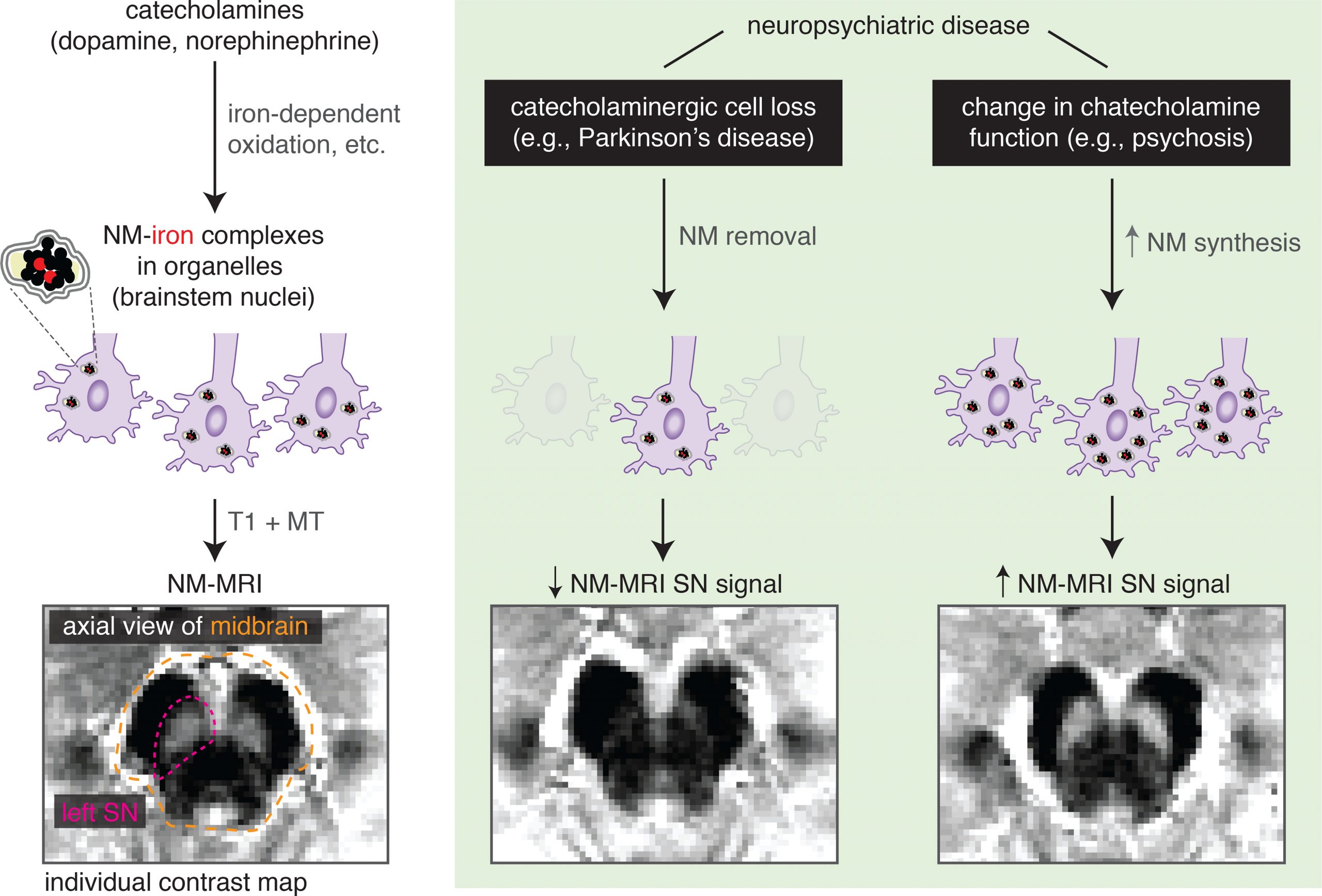
Neuromelanin-sensitive MRI is a non-invasive neuroimaging approach with an increasing number of applications in psychiatric research. This MRI modality is sensitive to the concentration of neuromelanin, which is synthesized from intracellular catecholamines and accumulates in catecholaminergic nuclei including the dopaminergic substantia nigra and the noradrenergic locus coeruleus. Emerging data suggest the utility of neuromelanin-sensitive MRI as a proxy measure for variability in catecholamine metabolism and function, even in the absence of catecholaminergic cell loss. Given the importance of catecholamine function to several psychiatric disorders and their treatments, neuromelanin-sensitive MRI is ideally positioned as an informative and ready-to-acquire catecholaminergic index. Our work has laid the foundation for developing clinical applications using neuromelanin-sensitive MRI through the development of advanced analysis methods, the optimization of acquisition and analysis, benchmarking its reliability, and developing a multi-site harmonization method. Furthermore, using cross-validated machine-learning analyses we have provided a proof-of-concept demonstration that multivoxel neuromelanin-sensitive MRI patterns can be used to predict psychosis severity in new data, suggesting potential for developing clinically useful tools. We aim to extend this work to larger samples and applications such as predicting treatment response to antipsychotics to facilitate earlier transition to clozapine.
Neuromelanin-sensitive MRI as a biomarker of catecholamine function in psychiatry

Neuromelanin-sensitive MRI is a non-invasive neuroimaging approach with an increasing number of applications in psychiatric research. This MRI modality is sensitive to the concentration of neuromelanin, which is synthesized from intracellular catecholamines and accumulates in catecholaminergic nuclei including the dopaminergic substantia nigra and the noradrenergic locus coeruleus. Emerging data suggest the utility of neuromelanin-sensitive MRI as a proxy measure for variability in catecholamine metabolism and function, even in the absence of catecholaminergic cell loss. Given the importance of catecholamine function to several psychiatric disorders and their treatments, neuromelanin-sensitive MRI is ideally positioned as an informative and ready-to-acquire catecholaminergic index. Our work has laid the foundation for developing clinical applications using neuromelanin-sensitive MRI through the development of advanced analysis methods, the optimization of acquisition and analysis, benchmarking its reliability, and developing a multi-site harmonization method. Furthermore, using cross-validated machine-learning analyses we have provided a proof-of-concept demonstration that multivoxel neuromelanin-sensitive MRI patterns can be used to predict psychosis severity in new data, suggesting potential for developing clinically useful tools. We aim to extend this work to larger samples and applications such as predicting treatment response to antipsychotics to facilitate earlier transition to clozapine.
