New tools for wireless neural modulation Tools for neural activation or silencing have enabled incredible advances in our understanding of the specific neural populations. Recently we developed a system for rapid, remote neural modulation in freely moving animals using static or alternating magnetic fields (radiowaves)4 (see video). This has been termed radiogenetics. We combine 1) static or alternating magnetic fields that freely penetrate tissue, 2) cell-type specific expression of a GFP-tagged iron storage protein, ferritin, to generate intracellular iron oxide nanoparticles and 3) a modified multimodal channel, transient receptor potential vanilloid 1 receptor (TRPV1)6. Static magnetic fields and radiowaves penetrate tissue with minimal absorption but interact with iron oxide nanoparticles, which absorb their energy. In the presence of either static or alternating magnetic fields, energy absorbed by ferritin is transferred to TRPV1 leading to channel opening, cation entry, depolarization and neural activation. Using this system, we can use radiowaves and magnetic field treatment to activate neruons in vitro and in vivo. We also modified our system to allow remote neural silencing by modifying TRPV1 to convert it to a chloride-conducting channel. When ferritin is tethered to this modified TRPV1, radiowave and magnet treatment now results in chloride entry, hyperpolarization and neural silencing in vitro and in vivo. These tools can be used to remotely activate or inhibit neurons in freely moving, untethered mice to regulate physiological functions. We are continuing to develop tools for remote neural modulation based on this system which will allow us to investigate the roles of specific neural populations in metabolism. CNS regulation of glucose metabolism Glucose is a key nutrient for brain and other organs and maintenance of glucose homeostasis is required for normal physiological function and health. Acute and chronic disruptions in glucose homeostasis produce considerable morbidity and mortality. Although a role for the CNS in peripheral metabolic regulation has been known since the work of Claude Bernard, many aspects remain poorly defined. Specialized neural populations in the CNS change their activity in response to altered extracellular glucose concentration. Glucose-sensing neurons in the CNS in turn regulate glucose metabolism by effects on insulin release and sensitivity, glucose synthesis and uptake along with hormone release, feeding behavior, and body temperature. Several CNS glucose sensing mechanisms have been reported. There is considerable evidence that the low affinity hexokinase, glucokinase (Gck), may act as a neural glucose sensor. It is expressed in many hypothalamic regions and elsewhere in the CNS. Our ongoing studies investigate the roles of glucose-sensing neurons in regulating glucose metabolism with the aim of identifying specific populations and pathways that may eventually lead to new therapies for diabetes. 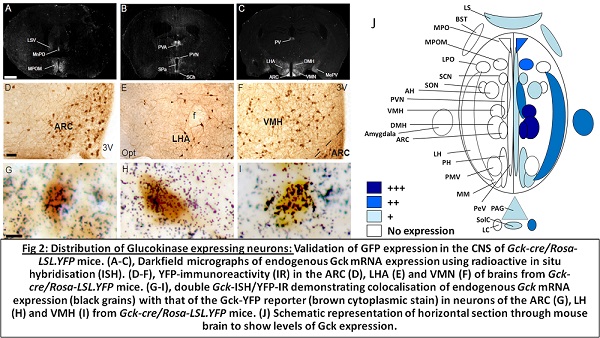 Funding 1. U01 MH105941 Stanley (PI) 09/26/14-06/30/17 Remote regulation of neural activity 2. ADA #7-14-JF-52 Stanley (PI) 07/01/15-06/30/18 The Role of Glucose-Sensing Neurons in the Ventromedial Hypothalamus in Physiological Regulation of Glucose Homeostasis, 3. Einstein-Mt. Sinai Diabetes Research Center Pilot and Feasibility award 05/31/16-30/5/17 Remote modulation of hypothalamic glucose sensing neurons to investigate glucose homeostasis. 4. 1R01NS097184-01 Bioengineering Research Partnerships (BRP) 07/01/16 – 07/01/21 Remote Electromagnetic Control of Neural Activity for Treatment of Parkinson’s Disease
Funding 1. U01 MH105941 Stanley (PI) 09/26/14-06/30/17 Remote regulation of neural activity 2. ADA #7-14-JF-52 Stanley (PI) 07/01/15-06/30/18 The Role of Glucose-Sensing Neurons in the Ventromedial Hypothalamus in Physiological Regulation of Glucose Homeostasis, 3. Einstein-Mt. Sinai Diabetes Research Center Pilot and Feasibility award 05/31/16-30/5/17 Remote modulation of hypothalamic glucose sensing neurons to investigate glucose homeostasis. 4. 1R01NS097184-01 Bioengineering Research Partnerships (BRP) 07/01/16 – 07/01/21 Remote Electromagnetic Control of Neural Activity for Treatment of Parkinson’s Disease
