To elucidate the importance of individual signaling pathways in mediating PDGF responses in vivo, we have generated allelic series at the Pdgfra and Pdgfrb loci in which docking sites for various effectors (PI3K, PLCγ, RASGAP, SHP-2, and SRC) have been mutated. Loss of one or several docking sites leads to hypomorphic mutations of increasing severity (Heuchel et al., 1999; Tallquist et al., 2000; Klinghoffer et al., 2002; Tallquist et al., 2003). To study the general question of the origin of receptor specificity, we have also studied phenotypic rescues resulting from kinase domain swaps between the two receptors (Klinghoffer et al., 2001). We have also examined the ability of two receptors of increasing divergence, Drosophila TORSO and the mouse FGFR1, to complement PDGF signaling (Hamilton et al., 2003).
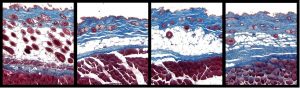
PDGF receptors are normally tightly regulated, so that they have very low basal activity in the absence of ligand. However, deregulation of the PDGF signaling pathway has been implicated in various physiological conditions and in disease, including cancer. To investigate the consequences of PDGF deregulation in the embryo and in the adult, we have generated knock-in alleles expressing constitutively active variants of Pdgfra and Pdgfrb in a conditional fashion. Activation of Pdgfra was thus shown to lead to frequent fibrosarcomas and generalized fibrosis. Both of these phenotypes were enhanced in the absence of INK4A/ARF, suggesting a new role for tumor suppressors in attenuating fibrotic diseases (Olson and Soriano, 2009). Conditional activation of Pdgfra in neural tissues leads to mixed gliomas (Zou et al., 2015). We have used this as a sensitized background to identify factors that cooperate with PDGF in glioma progression, using a gene trap approach combined with somatic transposon mutagenesis (Friedel at al., 2013). Consistent with key roles for PDGFRβ in vascular development, activation of this receptor regulates vascular smooth muscle cell plasticity, and prevents their differentiation. Increased PDGF signaling also changes the pericyte ground state to maintain mesenchymal progenitor status, resulting in regression of white adipose tissue while inducing a battery of immune response genes (Olson and Soriano, 2011).
