Refinement and application of high-resolution multi-contrast and quantitative ex vivo MRI methods to complement and enhance neuropathological research
MRI is an extremely valuable 3D non-destructive method for investigation of post-mortem tissue. Multiple pulse sequences can provide information on microstructural integrity of white matter, myelination, edema, infarction, and hemorrhage throughout the entire brain at high spatial resolution. However, MRI cannot yet deliver the level of microscopic detail and specificity that histology can provide. Although histology provides incredibly detailed and specific information on a microscopic level, this information is only available in tissue that is selected a priori for sectioning and staining. It is therefore very likely that sectioning and visual examination will fail to sample all abnormal regions in the brain, particularly small microhemorrhages or microinfarctions that are smaller than several millimeters. The integration of MRI and histology methods can combine the sensitivity of whole-brain 3D imaging with the specificity and detail of histopathological analysis. This renders the unification of MRI and histology more effective than the sum of the two methods in isolation.
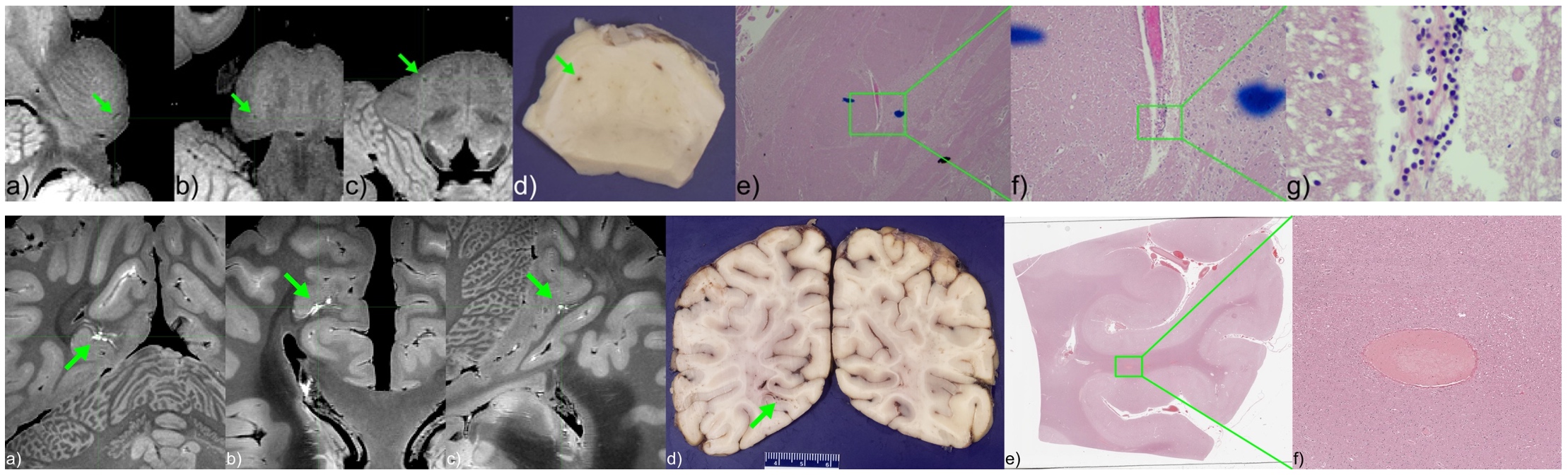
High-resolution ex vivo MRI of fixed tissue specimens can identify small lesions that are likely to be missed on standard neuropathological sampling.
Development of anatomical, functional, and diffusion MRI methods at ultra-high field (7 Tesla) to study neural circuits spanning the spinal cord, brainstem, cerebellum, and brain, particularly those involved in pain modulation
The cervical spinal cord and brainstem are vital central nervous system (CNS) structures that connect the forebrain to the body. Despite its critical role in wide range of functions, the human brainstem receives disproportionally less attention than the cerebrum or basal ganglia in in vivo MRI neuroimaging studies. The cervical spinal cord is simpler in structure than the brainstem, and its dysfunction is more clearly related to disability. In the past decade, cervical spinal cord MRI has found important applications in neurological disorders involving the spinal cord, such as multiple sclerosis, cervical spondylotic myelopathy, and spinal cord injury, with most of these imaging techniques developed for 3 Tesla (T). However, limited spatial resolution at 3T remains a critical barrier to advancing neuroimaging of small but important gray matter (GM) nuclei and white matter (WM) fasciculi in the cervical spinal cord and brainstem. The recently increasing availability of ultra-high field (7 Tesla) scanners heralds a new era of human cervical spinal cord and brainstem imaging. By leveraging the increased signal-to-noise ratio (SNR) and sensitivity to the blood oxygenation level-dependent (BOLD) effect at ultrahigh field, sufficient resolution is achievable to delineate fine structures and interpret function in a more meaningful way.
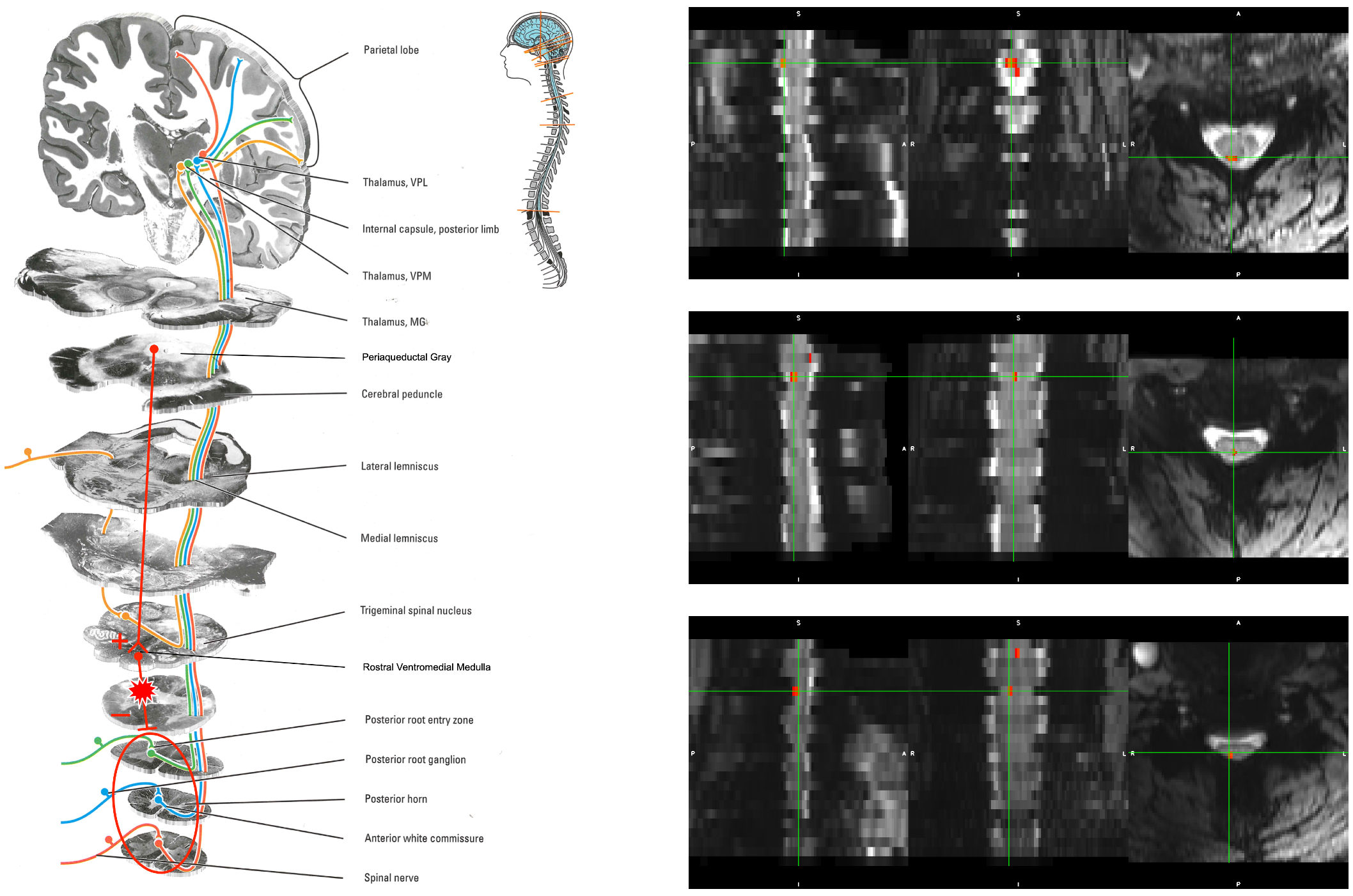
Sensory activation in response to thermal pain stimulation is visible in the human spinal cord at 7 T in individual subjects.
Development of solid-state MRI methods (ssMRI), such as ultrashort echo time (UTE) and zero echo time (ZTE), for direct quantification of myelin concentration
Myelin is a biomaterial composed of lipids, proteins, and water, that electrically insulates axons and ensures efficient action potential transduction. The concentration of myelin is closely related to the health of the nervous system, and deficient myelination and demyelination are hallmarks of several neurological disorders, such as multiple sclerosis, traumatic brain injury, and dementia. Non-invasive quantification of myelin content has important applications to the study, diagnosis, and monitoring of treatment of these debilitating disorders. Although myelin liquid crystalline 1H signal is not visible using conventional MRI pulse sequences due to its short transverse relaxation time (T2), quantitative magnetization transfer (qMT) and myelin water imaging (MWI) can infer myelin concentration via the interaction between water and myelin’s liquid crystalline lipid and protein components and have entered widespread use in human studies. Interaction with myelin, however, is not the only phenomenon affecting water 1H signal. This signal is affected by a host of biophysical phenomena, not all of which are fully understood or likely even discovered. Solid-state MRI methods, such as UTE and ZTE, can directly image the extremely short-T2 liquid crystalline 1H signal of myelin, and can provide a direct measurement of myelin concentration, unbiased by the vast range of effects modulating water 1H signal.
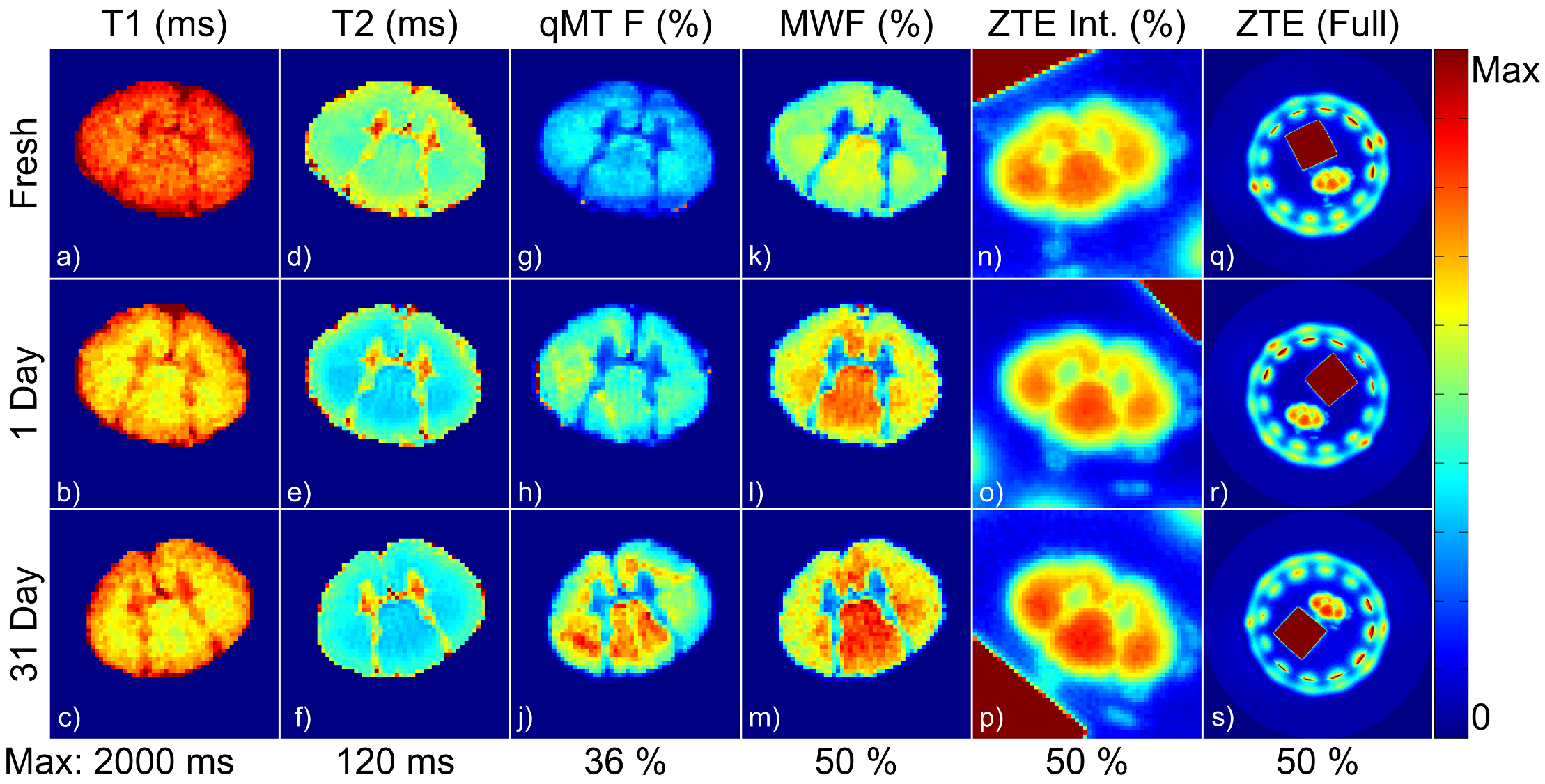
Formalin fixation significantly biases quantitative myelin measurements by indirect methods, such as myelin water imaging or quantitative magnetization transfer. Solid-state MRI-based methods, such as deuterium-exchanged ZTE, are more robust to formalin fixation.
