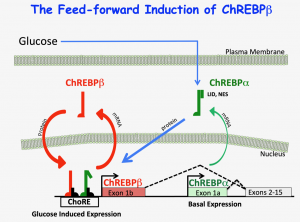
The Scott Lab has a longstanding interest in the molecular mechanisms by which nutrients act to change cellular phenotype. Cells must adapt to changes in the availability of metabolic fuel by altering networks of genes that control metabolism in a physiologically relevant manner. Dr. Scott has focused his interests on transcription factors that sense changes in glucose metabolism, including carbohydrate response element binding protein, (ChREBP), the anabolic transcription factor, Myc, and more recently the master regulator of antioxidant pathways, Nrf2. ChREBP is activated by glucose and promotes the expression of numerous genes involved in lipogenesis in the liver, adipose tissue and in numerous other tissues. Dr. Scott found that Myc is required for ChREBP activity. He used molecular approaches to demonstrate that depletion of Myc results in the inability of ChREBP to bind to glucose response elements or to transactivate target genes. Since glucose is a natural beta cell mitogen, and ChREBP is abundant in beta cells, Dr. Scott tested and confirmed the hypothesis that ChREBP is required for glucose-stimulated beta cell proliferation. He also recently demonstrated that the feed-forward induction of the constitutively active beta isoform of ChREBP is required for beta cell proliferation, but that too much ChREBP-beta, as might happen in diabetes, promotes apoptosis and is therefore a likely contributor to diabetic glucose toxicity. Furthermore, Dr. Scott has shown that overexpression of the regulatable form of ChREBP, ChREBP-alpha, stimulates a remarkable anabolic effect, promoting proliferation and resisting oxidative stress, providing a possible therapy for enhancing beta cell mass. He further demonstrated that activation of the Nrf2 pathway is necessary for normal, as well as ChREBP-augmented beta cell proliferation, and that activation of Nrf2 is sufficient to drive rodent and human beta cell proliferation in vitro, and that manipulation of the Nrf2 pathway controls beta cell replication in vivo. The Scott Lab continues to study the role of ChREBP isoforms in collaboration with Mark Herman at Duke University. In addition, in collaboration with Dr. Adolfo Garcia-Ocaña at Mount Sinai, Dr. Scott that induction of the anabolic, glucose-induced and stabilized transcription factor, Myc, is required for adaptive expansion of beta cell mass in response to an increased demand for insulin. In collaboration with Dr. Andrew Stewart and Dr. Martin Walsh at Mount Sinai, Dr. Scott is studying the 3-D structure of key chromatin regions involved in beta cell phenotype and proliferation using multidimensional –omic and chromatin conformation capture approaches. Also, along with Dr. Liora Katz, a Research Assistant Professor in the lab, Dr. Scott studies the role of T3 on ChREBP isoform expression. The relationships between Myc, ChREBP and Nrf2 are reflective of fundamental cellular adaptations to anabolic metabolic environments, which is applicable to a wide range of metabolic diseases, including diabetes, cancer, cardiovascular disease and aging.