The VCTL occupies over 1,200 square feet of space on the 5th floor of the Leon and Norma Hess Center for Science and Medicine. It provides a dedicated, controlled space to manufacture human therapeutics for Phase I and II clinical studies in accordance with current Good Manufacturing Practice (cGMP) and compliant with FDA regulations (CFR 21 Part 11, 210, and 211), that will ensure the safety, identity, purity and potency of the manufactured products.
VCTL operations are guided by over 100 Standard Operating Procedures (SOP), Master Batch Records (MBR), and Standard Forms (SF) documents that ensure adherence to applicable regulatory requirements and cGMP standards for manufacturing quality including facilities, equipment, personnel training, and work performance. A dedicated and independent Quality Assurance Specialist ensures compliance by conducting audits, inspection, and reviews and reports any issues to the Director of the VCTL.
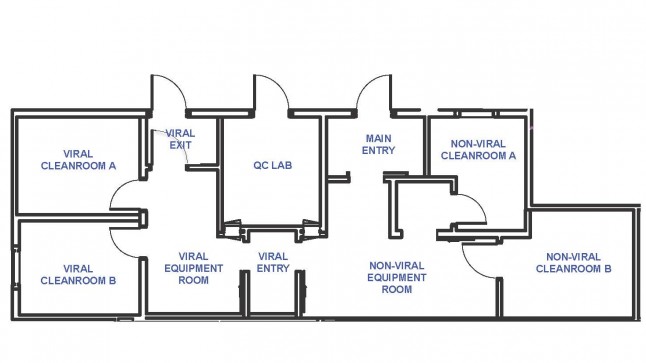
Design Features
- Controlled electronic card key access entrance
- ISO 7 and 8 classified rooms with single-pass HEPA filtered air
- Biosafety level 2 containment
- Separate non-viral and viral cleanrooms
- Separate non-viral and viral storage areas
- Unidirectional flow of personnel and materials with appropriate airlocks through viral cleanroom
- Manifold system for CO2, compressed air, and LN2
- Comprehensive environmental monitoring of equipment and room conditions using the Rees Monitoring System


Equipment
Cleanrooms
- Baker Class II Biosafety Cabinets
- Steri-Cult Incubators
- Miltenyi CliniMACS Prodigy
- Beckman L-80 XP ultracentrifuge
- Sorval XFR/XTR centrifuges
- Nexcelom Cellometer
- EVOS XL Core microscope
- Mettler Toledo XP analytical balance
- CryoMed Controlled Rate Freezers
- Storage: pharmacy refrigerator, -20°C, -80°C, and liquid nitrogen storage tank
QC Laboratory
- Attune NxT
- Baker Class II Biosafety Cabinets
- Steri-Cult Incubators
- Automated Cell Counters: Cellometer 2000
- EVOS XL Core microscope
- Sorval XFR/XTR centrifuges
- Charles River Endosafe
- Storage: pharmacy refrigerator, -20°C, -80°C, and liquid nitrogen storage tank
The adjacent Quality Control Laboratory has the capacity to perform in-process, lot release, validation and stability testing.
The dedicated Tissue Culture Laboratory is used for processing patient samples for biobanking as well as for performing immune-assays for correlative studies.
