
VCTL Services
VCTL was designed with the aim of supporting phase I and II clinical trials in cancer and other diseases. With this goal in view, experts at the VCTL work collaboratively with investigators and sponsors to help them initiate and conduct early phase clinical studies. These services include cGMP manufacturing (Viral and Non-Viral products), Analytical testing, Validation studies, Biobanking, Immune-analysis, etc.
Press Releases
“Mount Sinai Study Advances Understanding of Personalized Vaccines for Bladder Cancer”
View Here – Experimental vaccine, teamed with immunotherapy, shows safety, feasibility, and strong immune responses
“Personalized Cancer Vaccine Proves Promising in a Phase 1 Trial at Mount Sinai”
Facility Information
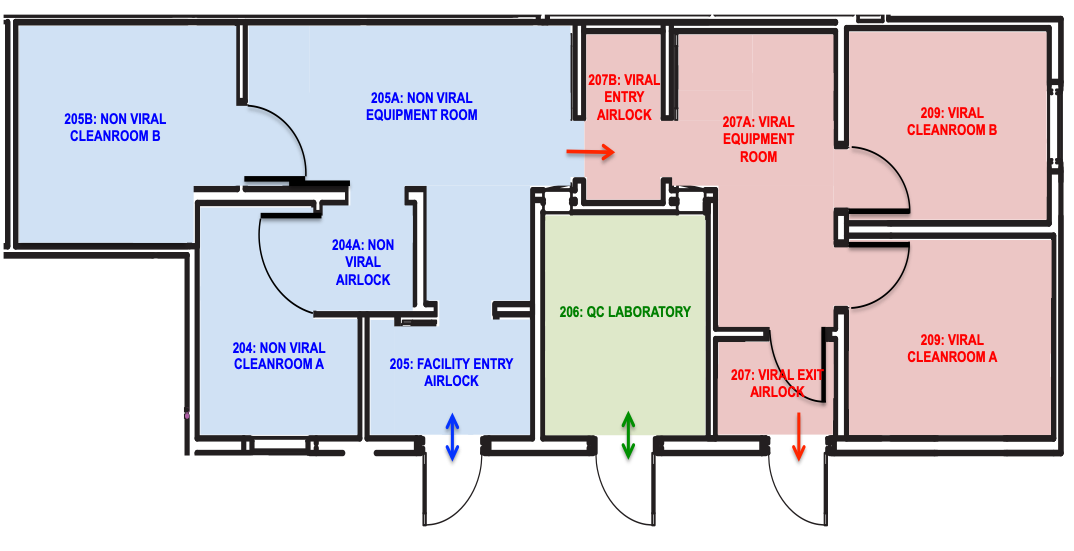
The VCTL occupies over 1,200 square feet of space on the 5th floor of the Leon and Norma Hess Center for Science and Medicine. It provides a dedicated, controlled space to manufacture human therapeutics for Phase I and II clinical studies in accordance with current Good Manufacturing Practice (cGMP) and compliant with FDA regulations (CFR 21 Part 11, 210, and 211), that will ensure the safety, identity, purity and potency of the manufactured products.

Highlighted Publications
PGV001, a Multi-Peptide Personalized Neoantigen Vaccine Platform
Saxena, M., Marron, T. U., Kodysh, J., Finnigan, J. P., Jr, Onkar, S., Kaminska, A., Tuballes, K., Guo, R., Sabado, R. L., Meseck, M., O’Donnell, T. J., Sebra, R. P., Parekh, S., Galsky, M. D., Blasquez, A., Gimenez, G., Bicak, M., Cimen Bozkus, C., Delbeau-Zagelbaum, D., Rodriguez, D., … Bhardwaj, N. (2025). PGV001, a Multi-Peptide Personalized Neoantigen Vaccine Platform: Phase I Study in Patients with Solid and Hematologic Malignancies in the Adjuvant Setting. Cancer discovery, 15(5), 930–947.
Atezolizumab plus personalized neoantigen vaccination in urothelial cancer: a phase 1 trial
Saxena, M., Anker, J. F., Kodysh, J., O’Donnell, T., Kaminska, A. M., Meseck, M., Hapanowicz, O., Niglio, S. A., Salazar, A. M., Shah, H. R., Kinoshita, Y., Brody, R., Rubinsteyn, A., Sebra, R. P., Bhardwaj, N., & Galsky, M. D. (2025). Atezolizumab plus personalized neoantigen vaccination in urothelial cancer: a phase 1 trial. Nature cancer, 10.1038/s43018-025-00966-7.
Therapeutic cancer vaccines
Saxena, M., van der Burg, S. H., Melief, C. J. M., & Bhardwaj, N. (2021).
Therapeutic cancer vaccines. Nature reviews. Cancer, 21(6), 360–378.
A T-cell-based immunogenicity protocol for evaluating human antigen-specific responses
Cimen Bozkus, C., Blazquez, A. B., Enokida, T., & Bhardwaj, N. (2021).
A T-cell-based immunogenicity protocol for evaluating human antigen-specific responses. STAR protocols, 2(3), 100758.
Flt3 ligand augments immune responses to anti-DEC-205-NY-ESO-1 vaccine through expansion of dendritic cell subsets
Bhardwaj, N., Friedlander, P. A., Pavlick, A. C., Ernstoff, M. S., Gastman, B. R., Hanks, B. A., Curti, B. D., Albertini, M. R., Luke, J. J., Blazquez, A. B., Balan, S., Bedognetti, D., Beechem, J. M., Crocker, A. S., D’Amico, L., Danaher, P., Davis, T. A., Hawthorne, T., Hess, B. W., Keler, T., … Fling, S. P. (2020).
Flt3 ligand augments immune responses to anti-DEC-205-NY-ESO-1 vaccine through expansion of dendritic cell subsets. Nature cancer, 1(12), 1204–1217.
VCTL Leadership

Nina Bardwaj, MD PhD
Principal Investigator, Bardwaj Lab
Learn more...
Dr. Bhardwaj is an immunologist who has made seminal contributions to human dendritic cell biology, specifically with respect to their isolation, subset discovery, immunobiology, antigen presenting function, and use as vaccine adjuvants in humans. Dr. Nina Bhardwaj is the Director of Immunotherapy, Medical Director of the Vaccine and Cell Therapy Laboratory, and Co-Director of the Cancer immunology Program at the Tisch Cancer Institute. Additionally, she is the Ward Coleman Chair in Cancer Research as well as a Professor of Medicine and Urology at the Icahn School of Medicine at Mount Sinai.

Mansi Saxena, PhD
Facility Director, Vaccine and Cell Therapy Laboratory
Learn more...
Dr. Mansi Saxena is an expert in cGMP production of cancer vaccines, cellular therapies, infectious disease vaccines; and has in depth knowledge of immune mechanisms regulating the tumor microenvironment. Dr. Saxena has been directly involved in development neoantigen peptide vaccine pipeline at Mount Sinai, PGV-001. Under this program, four clinical trials have been performed in various cancer setting, with more than 300 vaccines administered. In addition, Dr. Saxena directly oversaw the cGMP production of egg-based Newcastle Disease Virus based Covid-19 (NDV-HXP-S) vaccine Master Virus Seed that were used in several countries around the world. Notably, the live NDV-HXP-S vaccine manufactured at the VCTL was used in Phase-1 clinical trial at Mount Sinai, NY. Dr. Saxena also established the immune-monitoring program under the VCTL to investigate the immunogenicity of novel combinations of vaccines, immunotherapies and cell therapies in patients. Dr. Saxena has ably led the team since its inception in 2020 and currently the program serves as a single site for incisive cellular immune-monitoring services for several investigator initiated and externally sponsored trials at Mount Sinai. Dr. Saxena’s key areas of focus include development of novel cell therapies, monoclonal antibodies, vaccines and combination of immunotherapies to treat cancer and other disease and translate the same from bench to bedside.

Cansu Cimen Bozkus, PhD
Associate Director, Vaccine and Cell Therapy Laboratory
cansu.cimenbozkus@mssm.edu
Learn more...
Cancer Immunotherapy: 2023 Research and a Look Ahead with Nina Bhardwaj, MD, PhD
Challenges and barriers to using cancer vaccines in the clinic
Mansi Saxena, PhD, Icahn School of Medicine at Mount Sinai, New York, NY, comments on remaining unanswered questions regarding the development of cancer vaccines. Manufacturing costs remain high due to their personalized nature, and using shared neoantigens may led to off the shelf products, reducing cost. More advanced algorithms will additionally validate potential neoantigens, but achieving durable responses will be difficult using vaccines alone. This interview took place at the American Association for Cancer Research (AACR) Annual Meeting 2023 in Orlando, FL.
